Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 14(2); 2022 > Article
- Original Article Body Compositions of Elderly and Non-Elderly Patients Following Gastrectomy for Gastric Cancer
-
Ji Hoon Kang, M.D.1,2
 , Mi Ran Jung, M.D., Ph.D.1,2
, Mi Ran Jung, M.D., Ph.D.1,2 , Sung Eun Kim, M.D.1,2
, Sung Eun Kim, M.D.1,2 , Oh Jeong, M.D., Ph.D., F.A.C.S.1,2
, Oh Jeong, M.D., Ph.D., F.A.C.S.1,2
-
DOI: https://doi.org/10.15747/ACNM.2022.14.2.81
Published online: December 1, 2022
1Divsion of Gastroenterologic Surgery, Chonnam National University Hwasun Hospital, Hwasun, Korea
2Department of Surgery, Chonnam National University Medical School, Gwangju, Korea
- Corresponding author: Oh Jeong E-mail surgeonjeong@gmail.com ORCID https://orcid.org/0000-0002-7076-6998
© The Korean Society of Surgical Metabolism and Nutrition and The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 316 Views
- 1 Download
Abstract
-
Purpose Radical gastric resection is the mainstay of treatment in gastric cancer. However, patients can suffer from eating restrictions, weight loss, and malnutrition after gastrectomy, to which elderly patients are more vulnerable. We compared body composition changes in elderly patients and non-elderly patients after gastrectomy.
-
Materials and Methods This prospective study enrolled patients who underwent gastrectomy for gastric carcinoma between 2019 and 2021. Body composition was measured using bioelectrical impedance analysis (InBody S10) before surgery and up to 12 months after surgery. Patients were divided into an elderly group (>70 years) and a non-elderly group (≤70 years), and body composition changes were compared between the two groups using the linear mixed model.
-
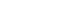
Results There were 69 patients in the elderly group and 164 patients in the non-elderly group. The groups showed no significant differences in gastric resection or pathologic stage. Overall, body composition, including total body water, body weight, lean body mass, skeletal muscle mass, and fat mass, decreased immediately after surgery and gradually improved until postoperative 12 months. A linear mixed model showed no significant time×group interactions for any body composition factors between groups.
-
Conclusion Body composition changes did not significantly differ between elderly patients and non-elderly patients after gastrectomy.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
AUTHOR CONTRIBUTIONS
Conceptualization: OJ. Data curation: JHK, SEK. Formal analysis: OJ, JHK. Funding acquisition: OJ. Investigation: OJ, JHK, MRJ, SEK. Methodology: OJ. Project administration: OJ. Resources: OJ. Supervision: MRJ. Validation: OJ, MRJ. Writing – original draft: JHK. Writing – review & editing: OJ, MRJ.
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
FUNDING
This study was supported by the research fund of the Korean Society of Surgical Metabolism and Nutrition (KSSMN) and a grant (HCRI 19016) from Chonnam National University Hospital Biomedical Research Institute.
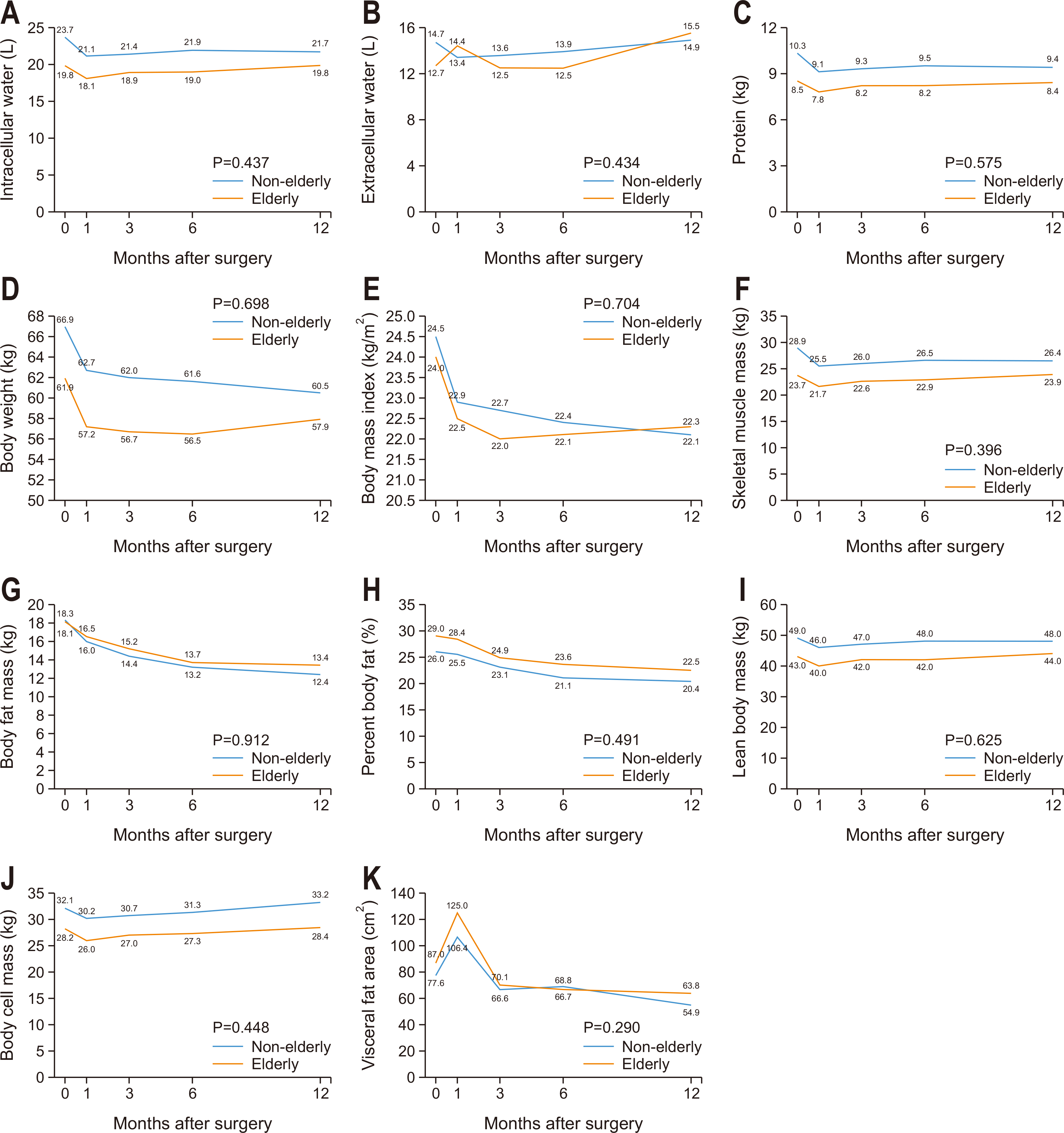
| Variable | Non-elderly (n=164) | Elderly (n=69) | P-value | |
|---|---|---|---|---|
| Age (yr) | 56.5±8.4 | 75.1±3.9 | <0.001 | |
| Sex | Female | 52 (31.7) | 25 (36.2) | 0.503 |
| Male | 112 (68.3) | 44 (63.8) | ||
| BMI (kg/m2) | 24.4±3.2 | 24.1±3.3 | 0.489 | |
| ASA status | 1 | 62 (37.8) | 5 (7.2) | <0.001 |
| 2 | 101 (61.6) | 52 (75.4) | ||
| 3 | 1 (0.6) | 12 (17.4) | ||
| Abdominal operation history | 18 (11.0) | 8 (11.6) | 0.891 | |
| Smoking | None | 83 (50.6) | 45 (65.2) | 0.009 |
| Current | 43 (26.2) | 6 (8.7) | ||
| Former | 35 (21.3) | 18 (26.1) | ||
| Operative approach | Open | 6 (3.7) | 6 (8.7) | 0.112 |
| Laparoscopy | 158 (96.3) | 63 (91.3) | ||
| Gastric resection | Distal | 139 (84.8) | 58 (84.1) | 0.893 |
| Total | 25 (15.2) | 11 (15.9) | ||
| Lymphadenectomy | D1+ | 134 (81.7) | 48 (69.6) | 0.041 |
| D2 | 30 (18.3) | 21 (30.4) | ||
| Reconstruction | Billroth II | 69 (42.1) | 44 (63.8) | 0.002 |
| Roux-en-Y | 95 (57.9) | 25 (36.2) | ||
| Omentectomy | Partial | 149 (90.9) | 57 (82.6) | 0.073 |
| Complete | 15 (9.1) | 12 (17.4) | ||
| Tumor size (cm) | 2.8±1.6 | 3.4±1.9 | 0.599 | |
| pTa | T1 | 127 (77.4) | 44 (63.8) | 0.098 |
| T2 | 18 (11.0) | 9 (13.0) | ||
| T3 | 11 (6.7) | 11 (15.9) | ||
| T4 | 8 (4.9) | 5 (7.2) | ||
| pNa | N0 | 138 (84.1) | 51 (73.9) | 0.250 |
| N1 | 12 (7.3) | 10 (14.5) | ||
| N2 | 7 (4.3) | 5 (7.2) | ||
| N3 | 7 (4.3) | 3 (4.3) | ||
| TNM stagea | Stage I | 135 (82.3) | 50 (72.5) | 0.236 |
| Stage II | 20 (12.2) | 13 (18.8) | ||
| Stage III | 9 (5.5) | 6 (8.7) | ||
Values are presented as mean±standard deviation.
ICW = intracellular water; ECW = extracellular water; BW = body weight; BMI = body mass index; SMM = skeletal muscle mass; BFM = body fat mass; PBF = percent body fat; LBM = lean body mass; BCM = body cell mass; BMC = bone mineral content; VFA = visceral fat area; BMR = basal metabolic rate.
- 1. Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol 2022;28:1187-203. ArticlePubMedPMC
- 2. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat 2020;52:335-50. ArticlePubMedPMCPDF
- 3. Kubota T, Shoda K, Konishi H, Okamoto K, Otsuji E. Nutrition update in gastric cancer surgery. Ann Gastroenterol Surg 2020;4:360-8. ArticlePubMedPMCPDF
- 4. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019;22:10-22; Erratum in: Gastric Cancer 2019;22:645-50. ArticlePubMedPDF
- 5. Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg 2005;9:313-9. ArticlePubMed
- 6. Aoyama T, Kawabe T, Hirohito F, Hayashi T, Yamada T, Tsuchida K, et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer 2016;19:645-50. ArticlePubMedPDF
- 7. Park KB, Park JY, Lee SS, Chung HY, Kwon OK. Chronological changes in quality of life and body composition after gastrectomy for locally advanced gastric cancer. Ann Surg Treat Res 2020;98:262-9. ArticlePubMedPMCPDF
- 8. Aoyama T, Sato T, Segami K, Maezawa Y, Kano K, Kawabe T, et al. Risk factors for the loss of lean body mass after gastrectomy for gastric cancer. Ann Surg Oncol 2016;23:1963-70. ArticlePubMedPDF
- 9. Park KB, Kwon OK, Yu W, Jang BC. Body composition changes after totally laparoscopic distal gastrectomy with delta-shaped anastomosis: a comparison with conventional Billroth I anastomosis. Surg Endosc 2016;30:4286-93. ArticlePubMedPDF
- 10. Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-led nationwide survey on surgically treated gastric cancers in 2019. J Gastric Cancer 2021;21:221-35. ArticlePubMedPMCPDF
- 11. Ramesh HS, Pope D, Gennari R, Audisio RA. Optimising surgical management of elderly cancer patients. World J Surg Oncol 2005;3:17.ArticlePubMedPMCPDF
- 12. Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer 2019;19:1-48; Erratum in: J Gastric Cancer 2019;19:372-3. ArticlePubMedPMCPDF
- 13. Jung MR, Ryu SY, Park YK, Jeong O. Compliance with an enhanced recovery after a surgery program for patients undergoing gastrectomy for gastric carcinoma: a phase 2 study. Ann Surg Oncol 2018;25:2366-73. ArticlePubMedPDF
- 14. Tonner PH, Kampen J, Scholz J. Pathophysiological changes in the elderly. Best Pract Res Clin Anaesthesiol 2003;17:163-77. ArticlePubMed
- 15. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016;19:986-93. ArticlePubMedPDF
- 16. Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 2015;593:4259-73. ArticlePubMedPMC
- 17. Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 2005;288:E883-91. ArticlePubMed
- 18. Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care 2003;6:21-9. PubMed
- 19. Aoyama T, Maezawa Y, Yoshikawa T, Segami K, Kano K, Hayashi T, et al. Comparison of weight and body composition after gastrectomy between elderly and non-elderly patients with gastric cancer. In Vivo 2019;33:221-7. ArticlePubMedPMC
- 20. Tweed TTT, van der Veen A, Tummers S, van Dijk DPJ, Luyer MDP, Ruurda JP, et al. Body composition is a predictor for postoperative complications after gastrectomy for gastric cancer: a prospective side study of the LOGICA trial. J Gastrointest Surg 2022;26:1373-87. ArticlePubMedPMCPDF
- 21. Aoyama T, Sato T, Hayashi T, Yamada T, Cho H, Ogata T, et al. Does a laparoscopic approach attenuate the body weight loss and lean body mass loss observed in open distal gastrectomy for gastric cancer? A single-institution exploratory analysis of the JCOG 0912 phase III trial. Gastric Cancer 2018;21:345-52. ArticlePubMedPDF
- 22. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Composition of the ESPEN Working Group. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr 2004;23:1226-43. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

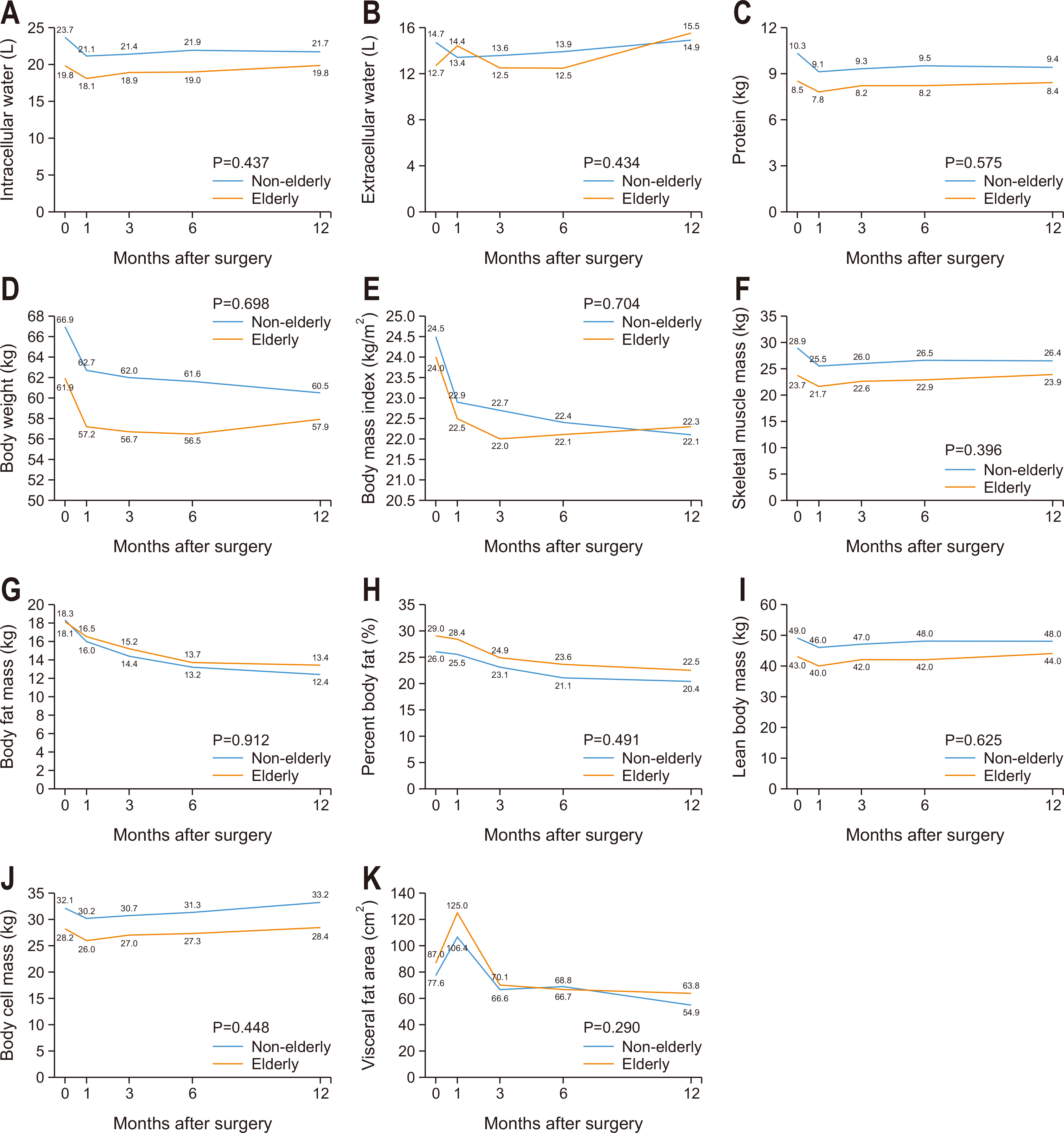
Fig. 1
Patient characteristics
| Variable | Non-elderly (n=164) | Elderly (n=69) | P-value | |
|---|---|---|---|---|
| Age (yr) | 56.5±8.4 | 75.1±3.9 | <0.001 | |
| Sex | Female | 52 (31.7) | 25 (36.2) | 0.503 |
| Male | 112 (68.3) | 44 (63.8) | ||
| BMI (kg/m2) | 24.4±3.2 | 24.1±3.3 | 0.489 | |
| ASA status | 1 | 62 (37.8) | 5 (7.2) | <0.001 |
| 2 | 101 (61.6) | 52 (75.4) | ||
| 3 | 1 (0.6) | 12 (17.4) | ||
| Abdominal operation history | 18 (11.0) | 8 (11.6) | 0.891 | |
| Smoking | None | 83 (50.6) | 45 (65.2) | 0.009 |
| Current | 43 (26.2) | 6 (8.7) | ||
| Former | 35 (21.3) | 18 (26.1) | ||
| Operative approach | Open | 6 (3.7) | 6 (8.7) | 0.112 |
| Laparoscopy | 158 (96.3) | 63 (91.3) | ||
| Gastric resection | Distal | 139 (84.8) | 58 (84.1) | 0.893 |
| Total | 25 (15.2) | 11 (15.9) | ||
| Lymphadenectomy | D1+ | 134 (81.7) | 48 (69.6) | 0.041 |
| D2 | 30 (18.3) | 21 (30.4) | ||
| Reconstruction | Billroth II | 69 (42.1) | 44 (63.8) | 0.002 |
| Roux-en-Y | 95 (57.9) | 25 (36.2) | ||
| Omentectomy | Partial | 149 (90.9) | 57 (82.6) | 0.073 |
| Complete | 15 (9.1) | 12 (17.4) | ||
| Tumor size (cm) | 2.8±1.6 | 3.4±1.9 | 0.599 | |
| pT |
T1 | 127 (77.4) | 44 (63.8) | 0.098 |
| T2 | 18 (11.0) | 9 (13.0) | ||
| T3 | 11 (6.7) | 11 (15.9) | ||
| T4 | 8 (4.9) | 5 (7.2) | ||
| pN |
N0 | 138 (84.1) | 51 (73.9) | 0.250 |
| N1 | 12 (7.3) | 10 (14.5) | ||
| N2 | 7 (4.3) | 5 (7.2) | ||
| N3 | 7 (4.3) | 3 (4.3) | ||
| TNM stage |
Stage I | 135 (82.3) | 50 (72.5) | 0.236 |
| Stage II | 20 (12.2) | 13 (18.8) | ||
| Stage III | 9 (5.5) | 6 (8.7) | ||
Values are presented as mean±standard deviation or number (%).
BMI = body mass index; ASA classification = American Society of Anesthesiologists physical status classification.
apTNM stages are based on the American Joint Committee on Cancer TNM classification, seventh edition.
Short-term surgical outcomes
| Variable | Non-elderly (n=164) | Elderly (n=69) | P-value |
|---|---|---|---|
| Operating time (min) | 180.6±63.6 | 163.5±56.8 | 0.055 |
| Operative blood loss (mL) | 35.5±62.0 | 30.7±25.8 | 0.531 |
| Morbidity | 19 (11.6) | 12 (17.4) | 0.234 |
| Local | 19 (11.6) | 11 (15.9) | 0.365 |
| Systemic | 2 (1.2) | 1 (1.4) | 0.887 |
| Mortality | 0 | 0 | |
| Harvested lymph nodes | 50.6±18.5 | 55.1±21.1 | 0.105 |
| Blood transfusion | 3 (1.8) | 1 (1.5) | 0.844 |
| Fever | 5 (3.0) | 5 (7.2) | 0.149 |
| Gas passage (day) | 2.7±1.0 | 2.6±0.8 | 0.148 |
| Diet resumption (day) | 1.8±2.1 | 1.7±1.6 | 0.701 |
| Hospital stay (day) | 7.5±2.0 | 7.5±1.5 | 0.914 |
Values are presented as mean±standard deviation or number (%).
Rates of change in body composition in elderly and non-elderly patients
| Variable | Baseline | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-elderly (n=164) | Elderly (n=69) | Non-elderly (n=143) | Elderly (n=61) | P-value | Non-elderly (n=135) | Elderly (n=48) | P-value | |||
| ICW (L) | 23.7±17.1 | 19.8±3.7 | –3.3±9.2 | –3.5±9.9 | 0.879 | –2.5±10.1 | –1.9±7.6 | 0.712 | ||
| ECW (L) | 14.7±10.1 | 12.7±2.4 | –1.7±8.4 | –0.9±8.0 | 0.543 | –0.6±10.5 | 0.1±7.0 | 0.654 | ||
| Protein (kg) | 10.3±7.4 | 8.5±1.6 | –3.4±8.8 | –2.7±8.7 | 0.593 | –2.8±9.5 | –3.4±14.2 | 0.756 | ||
| Mineral (kg) | 3.3±0.7 | 2.9±0.5 | –0.9±8.6 | –0.2 ±10.5 | 0.629 | 0.7±9.4 | 1.1±10.5 | 0.846 | ||
| BW (kg) | 66.9±11.2 | 61.9±12.4 | –9.27±11.3 | –8.3±11.7 | 0.579 | –9.0±6.5 | –8.0±10.5 | 0.449 | ||
| BMI (kg/m2) | 24.5±3.2 | 24.0±3.6 | –8.4±7.1 | –7.0±12.1 | 0.285 | –9.0±6.6 | –6.8±11.4 | 0.106 | ||
| SMM (kg) | 28.9±22.3 | 23.7±4.9 | –3.7±9.4 | –3.1±9.3 | 0.697 | –2.8±9.8 | –2.0±8.3 | 0.614 | ||
| BFM (kg) | 18.3±13.4 | 18.1±7.6 | –23.4±21.8 | –26.5±20.6 | 0.362 | –28.1±27.5 | –26.8±28.2 | 0.776 | ||
| PBF (%) | 26.0±8.7 | 29.0±8.0 | –16.8±21.6 | –17.1±23.5 | 0.928 | –20.9±25.5 | –21.6±27.1 | 0.874 | ||
| LBM (kg) | 49.5±10.0 | 43.8±8.2 | –2.3±5.4 | –2.1±8.4 | 0.826 | –1.5±6.1 | –1.1±7.4 | 0.718 | ||
| BCM (kg) | 32.1±6.6 | 28.2±5.4 | –2.9 ±5.6 | –2.9 ±8.6 | 0.962 | –2.0±6.2 | –1.8±7.7 | 0.854 | ||
| BMC (kg) | 2.7±0.6 | 2.4±0.4 | –0.7±9.3 | 0.4 ±10.9 | 0.489 | 1.2±9.9 | 1.8±10.7 | 0.744 | ||
| VFA (cm2) | 77.6±37.3 | 87.0±42.3 | –19.7±30.7 | –20.0±32.2 | 0.943 | –29.7±30.3 | –24.3±36.5 | 0.323 | ||
| BMR (kcal) | 1,448.6±177.9 | 1,316.0±177.9 | –1.7±7.5 | –1.5±6.1 | 0.823 | –1.8±11.5 | –0.8±5.3 | 0.534 | ||
Values are presented as mean±standard deviation.
ICW = intracellular water; ECW = extracellular water; BW = body weight; BMI = body mass index; SMM = skeletal muscle mass; BFM = body fat mass; PBF = percent body fat; LBM = lean body mass; BCM = body cell mass; BMC = bone mineral content; VFA = visceral fat area; BMR = basal metabolic rate.
Values are presented as mean±standard deviation or number (%). BMI = body mass index; ASA classification = American Society of Anesthesiologists physical status classification. apTNM stages are based on the American Joint Committee on Cancer TNM classification, seventh edition.
Values are presented as mean±standard deviation or number (%).
Values are presented as mean±standard deviation. ICW = intracellular water; ECW = extracellular water; BW = body weight; BMI = body mass index; SMM = skeletal muscle mass; BFM = body fat mass; PBF = percent body fat; LBM = lean body mass; BCM = body cell mass; BMC = bone mineral content; VFA = visceral fat area; BMR = basal metabolic rate.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 ePub Link
ePub Link Cite
Cite