Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 14(2); 2022 > Article
- Case Report Refeeding Syndrome after Gastrectomy in a Patient with Hypophosphatemia: A Case Report
-
Cheong Ah Oh, M.D.

-
DOI: https://doi.org/10.15747/ACNM.2022.14.2.88
Published online: December 1, 2022
Department of Hospital Medicine, Inha University Hospital, Incheon, Korea
- Corresponding author: Cheong Ah Oh E-mail caoh@inhauh.com ORCID https://orcid.org/0000-0002-1115-5150
© The Korean Society of Surgical Metabolism and Nutrition and The Korean Society for Parenteral and Enteral Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 386 Views
- 7 Download
Abstract
- This study describes an 81-year-old male who was highly suspicious for refeeding syndrome (RFS) after a retrospective review of his postoperative clinical progression. This highlights the importance of clinicians’ awareness of the possibility of RFS development in surgical patients with any RFS risk factors.
INTRODUCTION
CASE REPORT
CONCLUSION
CONFLICTS OF INTEREST
The author of this manuscript has no conflicts of interest to disclose.
FUNDING
None.
| Admission | Op day | POD 1 | POD 2 | POD 3 | POD 4 | |
|---|---|---|---|---|---|---|
| Infused caloric fluid | 5% DNK1 1,440 mL | 5% DNK1 1,440 mL | Winuf®peria 1,450 mL | 5% DNK1 1,440 mL | 5% DNK1 1,440 mL | 5% DNK1 1,440 mL |
| Total calorie (kcal) | 244.8 | 244.8 | 1,004 | 244.8 | 244.8 | 244.8 |
|
|
||||||
| Biochemical parameters (reference range) | ||||||
| White blood cell count (/μL) (4,000~10,000) | 3,960 | 9,110 | 14,830 | 16,470 | 14,250 | 8,960 |
| Hemoglobin (g/dL) (13.1~17.5) | 10.6 | 10.0 | 10.9 | 9.9 | 9.2 | 8.4 |
| Hematocrit (%) (39.0~52.0) | 35.2 | 31.8 | 35.9 | 31.3 | 28.9 | 26.2 |
| Platelet count (×1,000/μL) (140~400) | 166 | 146 | 127 | 90 | 62 | 56 |
| Na (mEq/l) (133~145) | NP | 138 | 135 | 136 | 134 | 133 |
| K (mEq/l) (3.5~5.5) | NP | 4.26 | 4.80 | 4.9 | 4.3 | 3.7 |
| Cl (mEq/l) (95~110) | NP | 105 | 103 | 107 | 104 | 102 |
| Calcium (mg/dL) (8.6~10.7) | 8.5 | 7.8 | 8.7 | 10.1 | 9.1 | 8.4 |
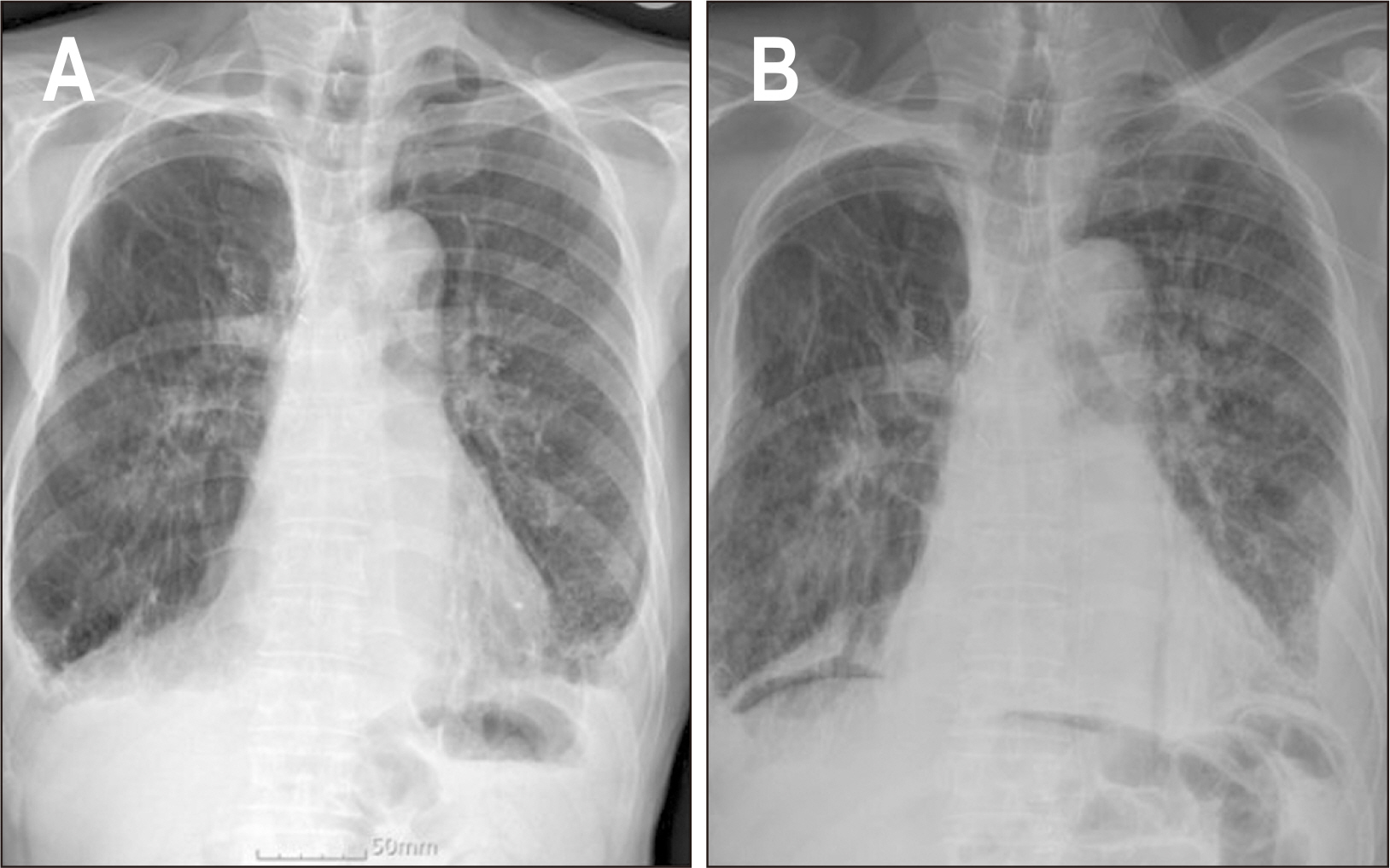
| Phosphate (mg/dL) (2.5~4.5) | 1.5 | 2.0 | 2.1 | 1.9 | 1.9 | 1.5 |
| Glucose (mg/dL) (70~100) | 89 | 114 | 157 | 98 | 124 | 114 |
| Albumin (mg/dL) (3.5~5.2) | 3.6 | 2.7 | 2.8 | 3.2 | 2.6 | 2.6 |
| BUN (mg/dL) (6~20) | 11.5 | 9.4 | 11.7 | 25.9 | 27.0 | 20.8 |
| Creatinine (mg/dL) (0.5~1.2) | 0.97 | 1.02 | 1.09 | 1.07 | 1.29 | 1.08 |
|
|
||||||
| Intake/output (mL/mL) | NP | 1,950/820 | 5,100/1,060 | 4,400/2,615 | 3,150/2,900 | 3,000/2,685 |
| Intraperitoneal drainage amount (mL) | 310 | 320 | 530 | 900 | 585 | |
|
|
||||||
| Injections | Hartmann’s solution Calcium gluconate 10% 40 mL 20% albumin 100 mL Volulyte®b 6% 500 mL | Hartmann’s solution Tamipool®c 1 ampoule Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g | Hartmann’s solution 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/tazobactam 4.5 g | Hartmann’s solution KH2PO4 20 mL 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/tazobactam 4.5 g | ||
|
|
||||||
| Body weight (kg) | 50.9 | 51.2 | 53.8 | 56.1 | 58.8 | 59.0 |
| Notable clinical features | HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | |||
| POD 5 | POD 6 | POD 7 | POD 9 | |
|---|---|---|---|---|
| Infused caloric fluid | 5% DNK1 1,440 mL | Nutriflex®peri 40a | ||
| Oral intake | SOW |
SFD Breakfast: whole meal (300 kcal) Lunch: 2/3 meal (200 kcal) Dinner: 1/4 meal (75 kcal) |
SBD (minimal) | None (intubation status) |
| Total calories (kcal) | 244.8 | 575 | <200 | 480 |
|
|
||||
| Biochemical parameters (reference range) | ||||
| White blood cell count (/μL) (4,000~1,000) | 7,480 | NP | 6,630 | 17,670 |
| Hemoglobin (g/dL) (13.1~17.5) | 9.4 | NP | 9.2 | 11.6 |
| Hematocrit (%) (39.0~52.0) | 29.2 | NP | 28.6 | 36.6 |
| Platelet count (×1,000/μL) (140~400) | 67 | NP | 94 | 142 |
| Na (mEq/l) (133~145) | 134 | NP | 132 | 135 |
| K (mEq/l) (3.5~5.5) | 3.6 | NP | 3.5 | 2.68 |
| Cl (mEq/l) (95~110) | 99 | NP | 95 | 95 |
| Calcium (mg/dL) (8.6~10.7) | 8.6 | NP | 8.0 | 8.3 |
| Phosphate (mg/dL) (2.5~4.5) | 2.0 | NP | 1.6 | 2.5 |
| Glucose (mg/dL) (70~100) | 92 | NP | 83 | 91 |
| Albumin (mg/dL) (3.5~5.2) | 3.1 | NP | 2.7 | 2.8 |
| BUN (mg/dL) (6~20) | 18.7 | NP | 13.7 | 23.5 |
| Creatinine (mg/dL) (0.5~1.2) | 1.06 | NP | 1.00 | 1.17 |
|
|
||||
| Intake/output (mL/mL) | 3,000/2,920 | 2,935/3,450 | 2,935/3,450 | 1,440/1,340 |
| Intraperitoneal drainage amount (mL) | 920 | 390 | 1,220 | 160 |
|
|
||||
| Injections | Hartmann’s solution Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g | Hartmann’s solution Tamipool®b 1 ampoule 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g Furosemide 30 mg | Hartmann’s solution KH2PO4 20 mL Tamipool®b 1 ampoule 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g Furosemide 20 mg | Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g |
|
|
||||
| Body weight (kg) | 50.9 | 51.2 | 53.8 | 56.1 |
| Notable clinical features | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion |
- 1. Lakananurak N, Gramlich L. The role of preoperative parenteral nutrition. Nutrients 2020;12:1320.ArticlePubMedPMC
- 2. Reber E, Friedli N, Vasiloglou MF, Schuetz P, Stanga Z. Management of refeeding syndrome in medical inpatients. J Clin Med 2019;8:2202.ArticlePubMedPMC
- 3. Ponzo V, Pellegrini M, Cioffi I, Scaglione L, Bo S. The refeeding syndrome: a neglected but potentially serious condition for inpatients. A narrative review. Intern Emerg Med 2021;16:49-60. ArticlePubMedPMCPDF
- 4. Buitendag J, Variawa S, Davids R, Ahmed N. Refeeding syndrome in surgical patients post initiation of artificial feeding, a prospective cohort study in a low-income country. Clin Nutr ESPEN 2021;46:210-5. ArticlePubMed
- 5. National Institute for Health and Care Excellence. 2006. London: United Kingdom: Available from: https://www.nice.org.uk/guidance/cg32/chapter/Recommendations#screening-for-malnutrition-and-the-risk-of-malnutrition-in-hospital-and-the-community. [cited 2022 May 24
- 6. Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr 2004;92:799-808. ArticlePubMed
- 7. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. ArticlePubMed
- 8. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. ArticlePubMed
- 9. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623-50. ArticlePubMed
- 10. Kim JW. Refeeding syndrome. J Clin Nutr 2015;7:15-22. Article
- 11. Friedli N, Stanga Z, Culkin A, Crook M, Laviano A, Sobotka L, et al. Management and prevention of refeeding syndrome in medical inpatients: an evidence-based and consensus-supported algorithm. Nutrition 2018;47:13-20. ArticlePubMed
- 12. Friedli N, Stanga Z, Sobotka L, Culkin A, Kondrup J, Laviano A, et al. Revisiting the refeeding syndrome: results of a systematic review. Nutrition 2017;35:151-60. ArticlePubMed
- 13. McKnight CL, Newberry C, Sarav M, Martindale R, Hurt R, Daley B. Refeeding syndrome in the critically ill: a literature review and clinician's guide. Curr Gastroenterol Rep 2019;21:58.ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

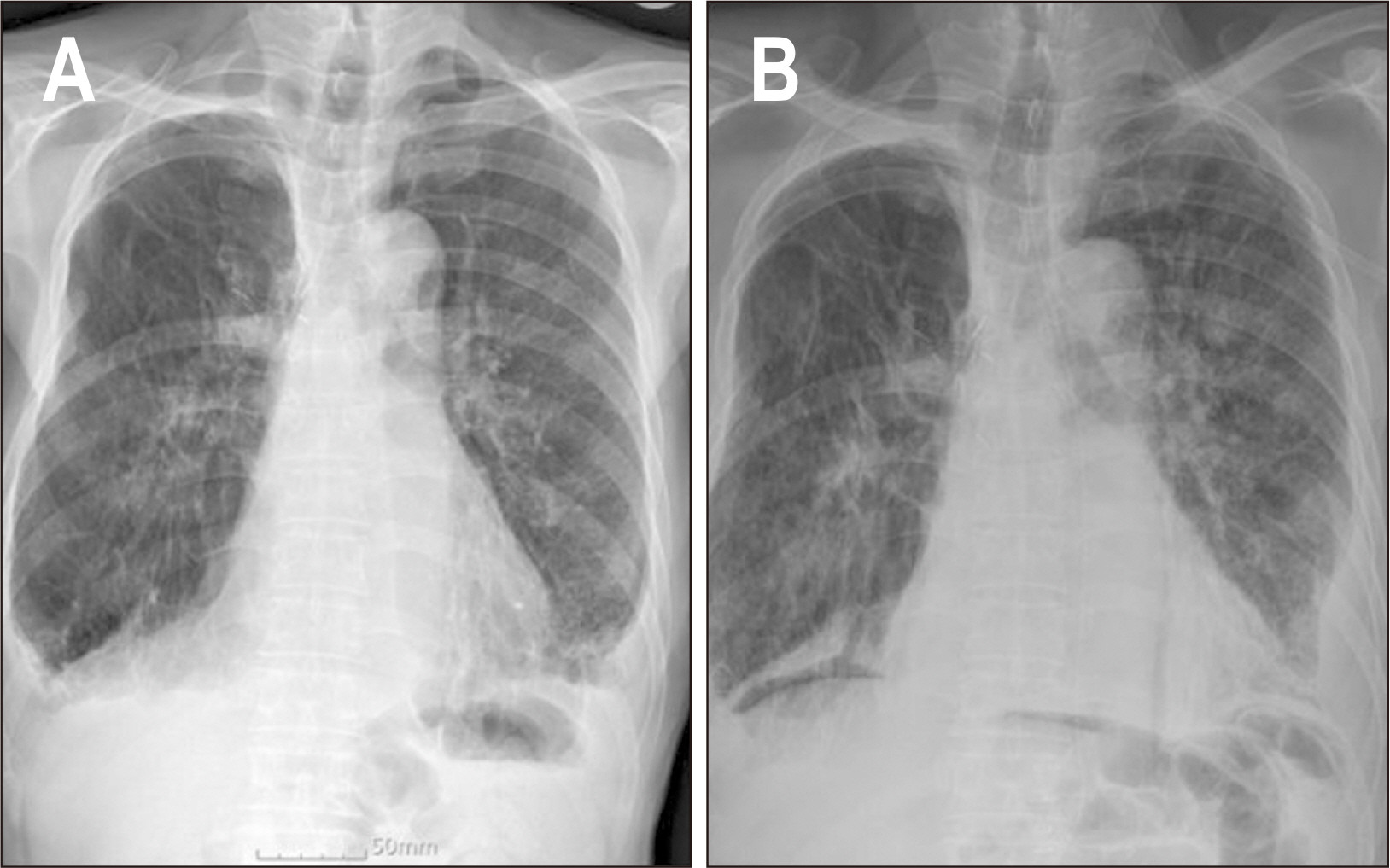
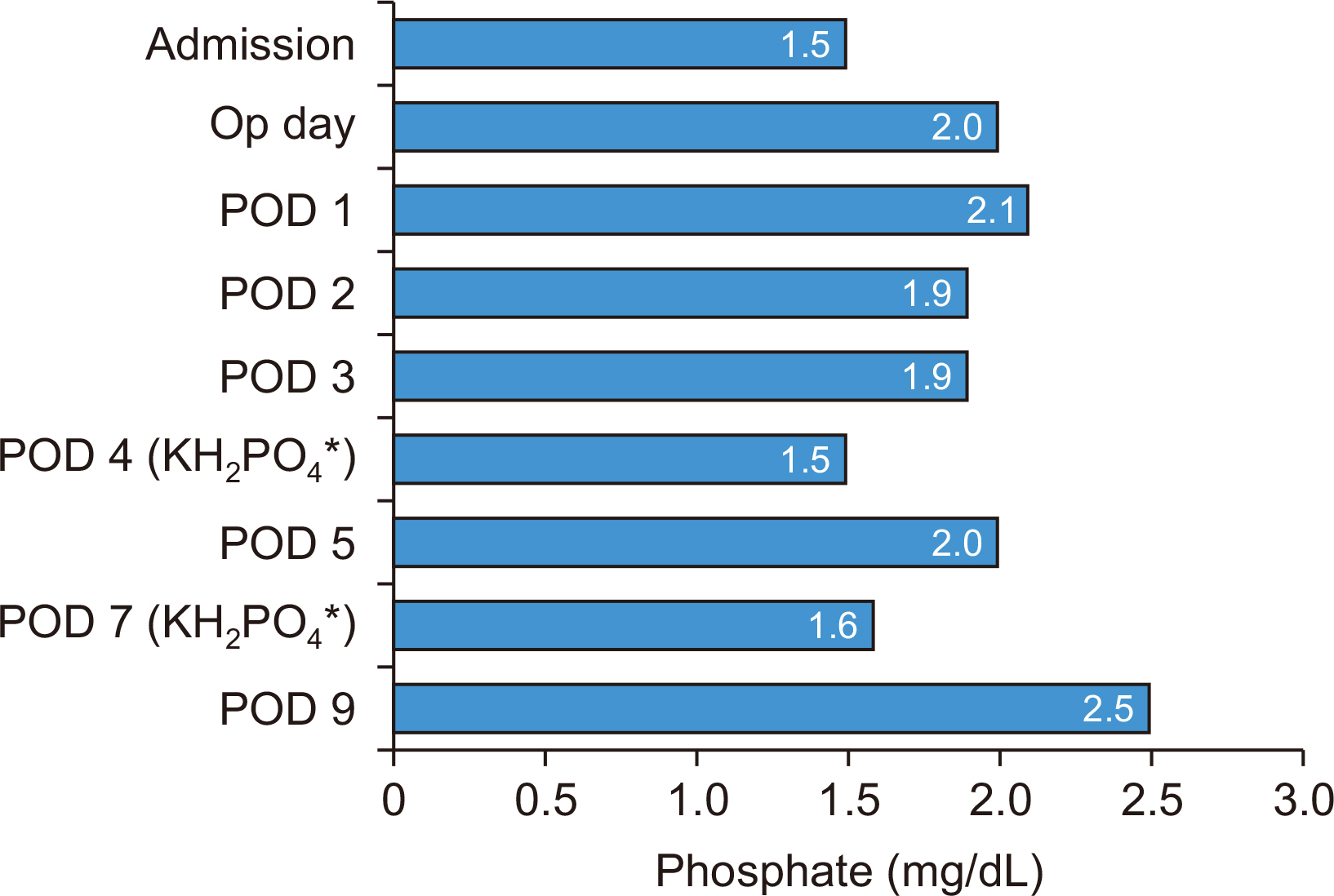
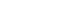
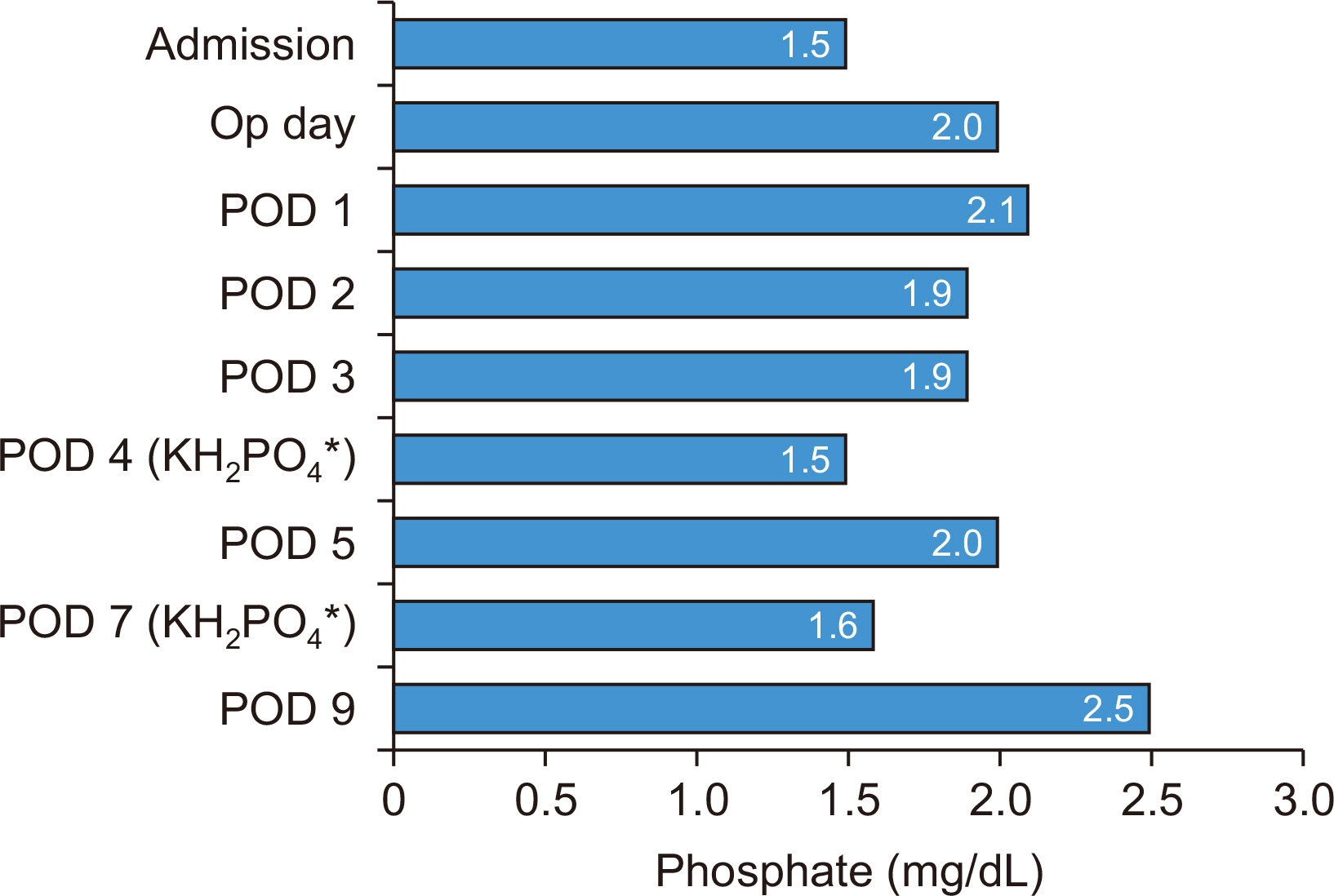
Fig. 1
Fig. 2
Laboratory test results and remarkable clinical characteristics according to net energy intake before resumption of oral intake
| Admission | Op day | POD 1 | POD 2 | POD 3 | POD 4 | |
|---|---|---|---|---|---|---|
| Infused caloric fluid | 5% DNK1 1,440 mL | 5% DNK1 1,440 mL | Winuf®peri |
5% DNK1 1,440 mL | 5% DNK1 1,440 mL | 5% DNK1 1,440 mL |
| Total calorie (kcal) | 244.8 | 244.8 | 1,004 | 244.8 | 244.8 | 244.8 |
| Biochemical parameters (reference range) | ||||||
| White blood cell count (/μL) (4,000~10,000) | 3,960 | 9,110 | 14,830 | 16,470 | 14,250 | 8,960 |
| Hemoglobin (g/dL) (13.1~17.5) | 10.6 | 10.0 | 10.9 | 9.9 | 9.2 | 8.4 |
| Hematocrit (%) (39.0~52.0) | 35.2 | 31.8 | 35.9 | 31.3 | 28.9 | 26.2 |
| Platelet count (×1,000/μL) (140~400) | 166 | 146 | 127 | 90 | 62 | 56 |
| Na (mEq/l) (133~145) | NP | 138 | 135 | 136 | 134 | 133 |
| K (mEq/l) (3.5~5.5) | NP | 4.26 | 4.80 | 4.9 | 4.3 | 3.7 |
| Cl (mEq/l) (95~110) | NP | 105 | 103 | 107 | 104 | 102 |
| Calcium (mg/dL) (8.6~10.7) | 8.5 | 7.8 | 8.7 | 10.1 | 9.1 | 8.4 |
| Phosphate (mg/dL) (2.5~4.5) | 1.5 | 2.0 | 2.1 | 1.9 | 1.9 | 1.5 |
| Glucose (mg/dL) (70~100) | 89 | 114 | 157 | 98 | 124 | 114 |
| Albumin (mg/dL) (3.5~5.2) | 3.6 | 2.7 | 2.8 | 3.2 | 2.6 | 2.6 |
| BUN (mg/dL) (6~20) | 11.5 | 9.4 | 11.7 | 25.9 | 27.0 | 20.8 |
| Creatinine (mg/dL) (0.5~1.2) | 0.97 | 1.02 | 1.09 | 1.07 | 1.29 | 1.08 |
| Intake/output (mL/mL) | NP | 1,950/820 | 5,100/1,060 | 4,400/2,615 | 3,150/2,900 | 3,000/2,685 |
| Intraperitoneal drainage amount (mL) | 310 | 320 | 530 | 900 | 585 | |
| Injections | Hartmann’s solution Calcium gluconate 10% 40 mL 20% albumin 100 mL Volulyte® |
Hartmann’s solution Tamipool® |
Hartmann’s solution 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/tazobactam 4.5 g | Hartmann’s solution KH2PO4 20 mL 20% albumin 100 mL Levofloxacin 750 mg Piperacillin/tazobactam 4.5 g | ||
| Body weight (kg) | 50.9 | 51.2 | 53.8 | 56.1 | 58.8 | 59.0 |
| Notable clinical features | HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | |||
Op = operation; POD = postoperative day; 5% DNK1 = dextrose 5% (187 kcal/bag); NP = not performed; HAP = hospital-acquired pneumonia.
aWinuf®peri (JW Pharmaceutical, Seoul, Korea); bVolulyte® (Fresenius Kabi Deutschland GmbH, Friedberg, Germany); cTamipool® (BCWP, Yeoju, Korea).
Laboratory test results and notable clinical features according to net energy intake after initiating oral intake
| POD 5 | POD 6 | POD 7 | POD 9 | |
|---|---|---|---|---|
| Infused caloric fluid | 5% DNK1 1,440 mL | Nutriflex®peri 40 |
||
| Oral intake | SOW | SFD Breakfast: whole meal (300 kcal) Lunch: 2/3 meal (200 kcal) Dinner: 1/4 meal (75 kcal) |
SBD (minimal) | None (intubation status) |
| Total calories (kcal) | 244.8 | 575 | <200 | 480 |
| Biochemical parameters (reference range) | ||||
| White blood cell count (/μL) (4,000~1,000) | 7,480 | NP | 6,630 | 17,670 |
| Hemoglobin (g/dL) (13.1~17.5) | 9.4 | NP | 9.2 | 11.6 |
| Hematocrit (%) (39.0~52.0) | 29.2 | NP | 28.6 | 36.6 |
| Platelet count (×1,000/μL) (140~400) | 67 | NP | 94 | 142 |
| Na (mEq/l) (133~145) | 134 | NP | 132 | 135 |
| K (mEq/l) (3.5~5.5) | 3.6 | NP | 3.5 | 2.68 |
| Cl (mEq/l) (95~110) | 99 | NP | 95 | 95 |
| Calcium (mg/dL) (8.6~10.7) | 8.6 | NP | 8.0 | 8.3 |
| Phosphate (mg/dL) (2.5~4.5) | 2.0 | NP | 1.6 | 2.5 |
| Glucose (mg/dL) (70~100) | 92 | NP | 83 | 91 |
| Albumin (mg/dL) (3.5~5.2) | 3.1 | NP | 2.7 | 2.8 |
| BUN (mg/dL) (6~20) | 18.7 | NP | 13.7 | 23.5 |
| Creatinine (mg/dL) (0.5~1.2) | 1.06 | NP | 1.00 | 1.17 |
| Intake/output (mL/mL) | 3,000/2,920 | 2,935/3,450 | 2,935/3,450 | 1,440/1,340 |
| Intraperitoneal drainage amount (mL) | 920 | 390 | 1,220 | 160 |
| Injections | Hartmann’s solution Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g | Hartmann’s solution Tamipool® |
Hartmann’s solution KH2PO4 20 mL Tamipool® |
Levofloxacin 750 mg Piperacillin/ tazobactam 4.5 g |
| Body weight (kg) | 50.9 | 51.2 | 53.8 | 56.1 |
| Notable clinical features | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion | Pitting edema HAP with bilateral pleural effusion |
POD = postoperative day; 5% DNK1 = dextrose 5% (187 kcal/bag); SOW = sips of water; SFD: semi-fluid diet; SBD = soft blended diet; NP = not performed; HAP = hospital-acquired pneumonia.
aNutriflex®peri 40 (B. Braun Medical AG, Crissier, Switzerland); bTamipool® (BCWP, Yeoju, Korea).
Op = operation; POD = postoperative day; 5% DNK1 = dextrose 5% (187 kcal/bag); NP = not performed; HAP = hospital-acquired pneumonia. aWinuf®peri (JW Pharmaceutical, Seoul, Korea); bVolulyte® (Fresenius Kabi Deutschland GmbH, Friedberg, Germany); cTamipool® (BCWP, Yeoju, Korea).
POD = postoperative day; 5% DNK1 = dextrose 5% (187 kcal/bag); SOW = sips of water; SFD: semi-fluid diet; SBD = soft blended diet; NP = not performed; HAP = hospital-acquired pneumonia. aNutriflex®peri 40 (B. Braun Medical AG, Crissier, Switzerland); bTamipool® (BCWP, Yeoju, Korea).

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN
 ePub Link
ePub Link Cite
Cite