Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 17(1); 2025 > Article
- Original Article Comparison of efficacy of enteral versus parenteral nutrition in patients after esophagectomy in Malaysia: a prospective cohort study
-
Ramizah Mohd Shariff1
 , Sze Chee Tee1
, Sze Chee Tee1 , Shukri Jahit Mohammad1
, Shukri Jahit Mohammad1 , Khei Choong Khong2
, Khei Choong Khong2
-
Annals of Clinical Nutrition and Metabolism 2025;17(1):41-49.
DOI: https://doi.org/10.15747/ACNM.24.016
Published online: April 1, 2025
1Department of Surgery, National Cancer Institute, Putrajaya, Malaysia
2Department of Pharmacy, National Cancer Institute, Putrajaya, Malaysia
- Corresponding author: Ramizah Mohd Shariff, email: skyzlimit49@gmail.com
© 2025 Korean Society of Surgical Metabolism and Nutrition · Korean Society for Parenteral and Enteral Nutrition · Asian Society of Surgical Metabolism and Nutrition · Japanese Society for Surgical Metabolism and Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,572 Views
- 67 Download
- 1 Crossref
Abstract
-
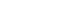
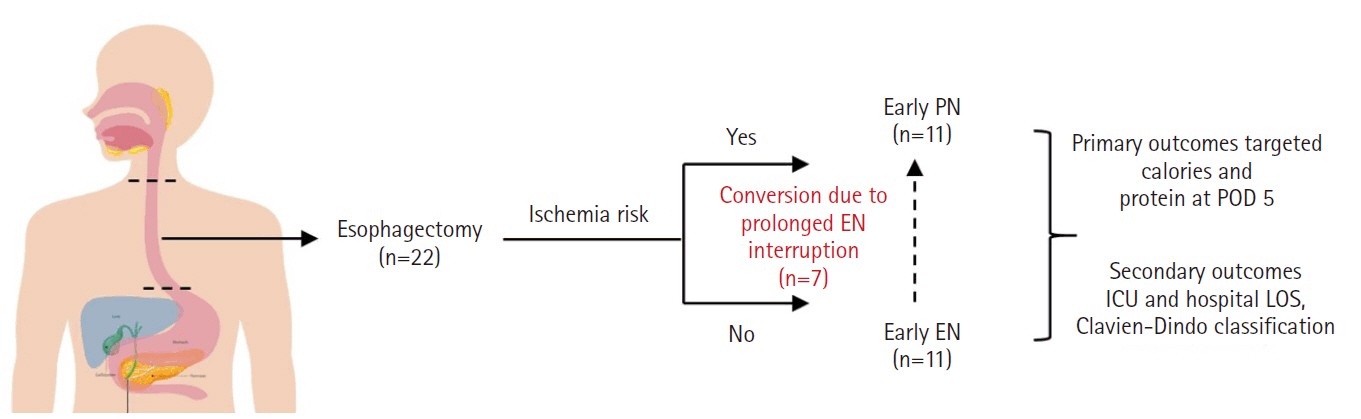
Purpose This study aims to assess the effectiveness of enteral versus parenteral feeding in patients after esophagectomy.
-
Methods This a prospective cohort study of post-esophagectomy intensive care unit (ICU) patients over 12 months in the National Cancer Institute, Malaysia. Early enteral feeding followed the Enhanced Recovery After Surgery protocol, and parenteral nutrition (PN) was considered if there was a risk for conduit ischemia. It compared the effectiveness of enteral versus PN following esophagectomy, and assessed the correlations between biochemical nutritional markers and hospital lengths of stay or ventilation days.
-
Results It included two cohorts receiving PN (n=11) or enteral nutrition (EN) (n=11) following elective esophagectomy. Preoperative weight, body mass index, and Subjective Global Assessment were higher in the EN group (P=0.033, P=0.021, P=0.031, respectively). Nutritional interruption occurred more frequently in the EN group (63.7%) compared to the PN group (P=0.001). Mean levels of energy and protein received were 93.1 kcal/kg and 1.4 g/kg for PN versus 92.4 kcal/kg and 1.2 g/kg for EN (P=0.893 and P=0.036). The median lengths of ICU stay (P=0.688) and postoperative stay (P=0.947) between groups showed no significant difference. In addition, 30-day mortality (P=0.214) and other postoperative complications (P>0.05) were comparable in the two groups.
-
Conclusion Early initiation of supplementary PN due to significant interruption in EN led to higher protein intake compared to the EN group. However, there were no significant differences in postoperative outcomes, including 30-day mortality, ICU length of stay, and ventilation days. PN ensures adequate nutritional intake, especially in terms of protein delivery, without adversely affecting postoperative recovery and clinical outcomes.
Graphical abstract
Introduction
Methods
Results
Discussion
Authors’ contribution
Conceptualization: all authors. Data curation; Formal analysis: RMS, KCK. Project administration: all authors. Funding acquisition: Not applicable. Writing – original draft: RMS. Writing – review & editing: TSC, MSJ. All authors read and approved the final manuscript.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
We sincerely thank the intensive care unit staff and the surgical team for their unwavering support and cooperation throughout the duration of this study. Additionally, we extend our gratitude to the patients and their families for their participation and valuable contributions.
Supplementary materials
None.
PN, parenteral nutrition; EN, enteral nutrition; SD, standard deviation; ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group; SGA, Subjective Global Assessment; BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; SSI, surgical site infection.
aIndependent t-test was applied, significant P-value was set at 0.05.
bChi-square test was applied, significant P-value was set at 0.05.
cFisher test was applied, significant P-value was set at 0.05.
| Biochemical parameter | Mean±SD | t-test (df) | P-valuea | |
|---|---|---|---|---|
| Non-pneumonia (n=10) | Pneumonia (n=12) | |||
| Albumin (g/L) | 27.8±2.62 | 29.2±3.30 | –1.06 (20) | 0.302 |
| CRP (mg/L) | 137.4±96.58 | 243.5±82.69 | –2.77 (20) | 0.012 |
| Correlation | Correlation coefficient | P-valuea |
|---|---|---|
| LOS vs. albumin | –0.256 | 0.250 |
| LOS vs. CRP | 0.424 | 0.049 |
| Ventilation days vs. albumin | –0.190 | 0.397 |
| Ventilation days vs. CRP | 0.276 | 0.213 |
- 1. Riccardi D, Allen K. Nutritional management of patients with esophageal and esophagogastric junction cancer. Cancer Control 1999;6:64-72. ArticlePubMedPDF
- 2. Mulazzani GE, Corti F, Della Valle S, Di Bartolomeo M. Nutritional support indications in gastroesophageal cancer patients: from perioperative to palliative systemic therapy: a comprehensive review of the last decade. Nutrients 2021;13:2766.ArticlePubMedPMC
- 3. Berkelmans GH, van Workum F, Weijs TJ, Nieuwenhuijzen GA, Ruurda JP, Kouwenhoven EA, et al. The feeding route after esophagectomy: a review of literature. J Thorac Dis 2017;9(Suppl 8):S785-91. ArticlePubMedPMC
- 4. Zheng R, Devin CL, Pucci MJ, Berger AC, Rosato EL, Palazzo F. Optimal timing and route of nutritional support after esophagectomy: a review of the literature. World J Gastroenterol 2019;25:4427-36. ArticlePubMedPMC
- 5. Peng J, Cai J, Niu ZX, Chen LQ. Early enteral nutrition compared with parenteral nutrition for esophageal cancer patients after esophagectomy: a meta-analysis. Dis Esophagus 2016;29:333-41. ArticlePubMed
- 6. Yu HM, Tang CW, Feng WM, Chen QQ, Xu YQ, Bao Y. Early enteral nutrition versus parenteral nutrition after resection of esophageal cancer: a retrospective analysis. Indian J Surg 2017;79:13-8. ArticlePubMedPMCPDF
- 7. Jeejeebhoy KN. Enteral nutrition versus parenteral nutrition: the risks and benefits. Nat Clin Pract Gastroenterol Hepatol 2007;4:260-5. ArticlePubMedPDF
- 8. Worthington P, Balint J, Bechtold M, Bingham A, Chan LN, Durfee S, et al. When is parenteral nutrition appropriate? JPEN J Parenter Enteral Nutr 2017;41:324-77. ArticlePubMedPDF
- 9. Fell DM, Bitetto EA, Skillman HE. Timing of enteral nutrition and parenteral nutrition in the PICU. Nutr Clin Pract 2023;38 Suppl 2:S174-212. ArticlePubMed
- 10. Mudge L, Isenring E, Jamieson GG. Immunonutrition in patients undergoing esophageal cancer resection. Dis Esophagus 2011;24:160-5. ArticlePubMed
- 11. Chow R, Bruera E, Chiu L, Chow S, Chiu N, Lam H, et al. Enteral and parenteral nutrition in cancer patients: a systematic review and meta-analysis. Ann Palliat Med 2016;5:30-41. ArticlePubMed
- 12. Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, Ruurda JP, van Hillegersberg R, Soeters PB, et al. Routes for early enteral nutrition after esophagectomy: a systematic review. Clin Nutr 2015;34:1-6. ArticlePubMed
- 13. Martinez-Ortega AJ, Pinar-Gutierrez A, Serrano-Aguayo P, Gonzalez-Navarro I, Remon-Ruiz PJ, Pereira-Cunill JL, et al. Perioperative nutritional support: a review of current literature. Nutrients 2022;14:1601.ArticlePubMedPMC
- 14. Rinninella E, Persiani R, D'Ugo D, Pennestrì F, Cicchetti A, Di Brino E, et al. NutriCatt protocol in the Enhanced Recovery After Surgery (ERAS) program for colorectal surgery: the nutritional support improves clinical and cost-effectiveness outcomes. Nutrition 2018;50:74-81. ArticlePubMed
- 15. Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr 2001;74:534-42. ArticlePubMed
- 16. Sadique Z, Harrison DA, Bear DE, Rowan KM, Grieve R; CALORIES Trial Investigators. Effectiveness and cost-effectiveness of early nutritional support via the parenteral versus the enteral route for critically ill adult patients. J Crit Care 2019;52:237-41. ArticlePubMed
- 17. Stratton RJ, Elia M. A review of reviews: a new look at the evidence for oral nutritional supplements in clinical practice. Clin Nutr Suppl 2007;2:5-23. Article
- 18. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623-50. ArticlePubMed
- 19. Nederlof N, de Jonge J, de Vringer T, Tran TC, Spaander MC, Tilanus HW, et al. Does routine endoscopy or contrast swallow study after esophagectomy and gastric tube reconstruction change patient management? J Gastrointest Surg 2017;21:251-8. ArticlePubMedPMCPDF
- 20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. ArticlePubMedPMC
- 21. Mehta CR, Patel NR. IBM SPSS exact tests. IBM Corporation; 2011.Article
- 22. Altman DG. Practical statistics for medical research. Chapman and Hall/CRC; 1990.Article
- 23. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ 1995;310:170.ArticlePubMedPMC
- 24. Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer 2009;45:3149-55. ArticlePubMed
- 25. Kutsogiannis J, Alberda C, Gramlich L, Cahill NE, Wang M, Day AG, et al. Early use of supplemental parenteral nutrition in critically ill patients: results of an international multicenter observational study. Crit Care Med 2011;39:2691-9. ArticlePubMed
- 26. Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117.ArticlePubMedPMC
- 27. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. ArticlePubMed
- 28. Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, et al. Monitoring nutrition in the ICU. Clin Nutr 2019;38:584-93. ArticlePubMed
- 29. Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130-8. ArticlePubMed
- 30. Wang WP, Yan XL, Ni YF, Guo K, Ke CK, Cheng QS, et al. Effects of lipid emulsions in parenteral nutrition of esophageal cancer surgical patients receiving enteral nutrition: a comparative analysis. Nutrients 2013;6:111-23. ArticlePubMedPMC
- 31. Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med 1999;17:1019-25. ArticlePubMed
- 32. Pokharel N, Katwal G, Adhikari SK. Comparison of preoperative nutritional risk index and body mass index for predicting immediate postoperative outcomes following major gastrointestinal surgery: cohort-study. Ann Med Surg (Lond) 2019;48:53-8. ArticlePubMedPMC
- 33. Pontiroli AE, Loreggian L, Rovati MP, De Patto E, Folini L, Raveglia F, et al. Length of hospitalization is associated with selected biomarkers (albumin and lymphocytes) and with co-morbidities: study on 4000 patients. Biomark Res 2017;5:13.ArticlePubMedPMCPDF
- 34. Lightdale J, Valim C, Newburg A, Heard L, Zgleszewski S, Fox V. Patient ratings by endoscopy unit providers using the American Society of Anesthesiologists (ASA) physical status classification scale: 122. J Pediatr Gastroenterol Nutr 2005;41:530-1. Article
- 35. Fietkau R, Lewitzki V, Kuhnt T, Holscher T, Hess CF, Berger B, et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer 2013;119:3343-53. ArticlePubMed
- 36. Deftereos I, Kiss N, Isenring E, Carter VM, Yeung JM. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol 2020;46:1423-34. ArticlePubMed
- 37. Reignier J, Boisrame-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133-43. ArticlePubMed
- 38. Ishii K, Tsubosa Y, Niihara M, Akai T, Soneda W. Changes in the nutritional status of elderly patients after esophagectomy. Esophagus 2019;16:408-12. ArticlePubMedPMCPDF
- 39. Kuzu MA, Terzioglu H, Genc V, Erkek AB, Ozban M, Sonyurek P, et al. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J Surg 2006;30:378-90. ArticlePubMedPDF
- 40. Takesue T, Takeuchi H, Ogura M, Fukuda K, Nakamura R, Takahashi T, et al. A prospective randomized trial of enteral nutrition after thoracoscopic esophagectomy for esophageal cancer. Ann Surg Oncol 2015;22 Suppl 3:S802-9. ArticlePubMedPDF
References
Figure & Data
REFERENCES
Citations

- Optimizing nutritional support in upper gastrointestinal surgery: A comprehensive review of feeding jejunostomy techniques and outcomes
Ioana Alexandra Prisacariu, Konstantinos Eleftherios Koumarelas, Konstantinos Argyriou, Alexandros Charalabopoulos, Grigorios Christodoulidis
World Journal of Gastrointestinal Surgery.2025;[Epub] CrossRef
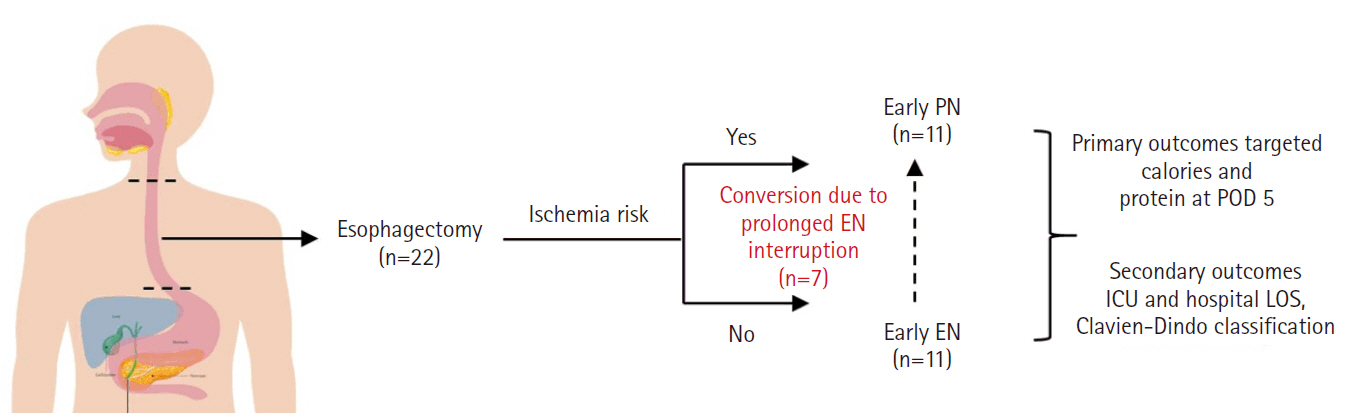
Fig. 1.
Graphical abstract
| Characteristic | All (n=22) | PN (n=11) | EN (n=11) | P-value |
|---|---|---|---|---|
| Age (yr), mean±SD | 58.0±12.79 | 60.9±10.63 | 55.1±14.55 | 0.297a |
| Sex, No. (%) | 0.647b | |||
| Male | 15 (68.2) | 7 (63.6) | 8 (72.7) | |
| Female | 7 (31.8) | 4 (36.4) | 3 (27.3) | |
| ASA, No. (%) | 0.540b | |||
| I | 7 (31.8) | 3 (27.3) | 4 (36.4) | |
| II | 11 (50.0) | 5 (45.5) | 6 (54.5) | |
| III | 4 (18.2) | 3 (27.3) | 1 (9.1) | |
| ECOG, No. (%) | 0.078b | |||
| 0 | 12 (54.5) | 4 (36.4) | 8 (72.7) | |
| 1 | 9 (40.9) | 7 (63.6) | 2 (18.2) | |
| 2 | 1 (4.5) | 0 | 1 (9.1) | |
| SGA, No. (%) | 0.031b | |||
| A | 10 (45.4) | 2 (18.2) | 8 (72.7) | |
| B | 6 (27.3) | 5 (45.5) | 1 (9.1) | |
| C | 6 (27.3) | 4 (36.4) | 2 (18.2) | |
| Stage, No. (%) | 0.189b | |||
| II | 2 (9.1) | 0 | 2 (18.2) | |
| III | 14 (63.7) | 9 (81.8) | 5 (45.5) | |
| III | 1 (4.5) | 0 (0.0) | 1 (9.1) | |
| IV | 1 (4.5) | 1 (9.1) | 0 | |
| NA | 4 (18.2) | 1 (9.1) | 2 (27.3) | |
| Weight (kg), mean±SD | 62.1±12.33 | 56.6±10.88 | 67.8±11.60 | 0.033a |
| BMI (kg/m2), mean±SD | 23.3±3.97 | 21.4±2.73 | 25.2±4.21 | 0.021a |
| ICU stay (day), median (IQR) | 3.0 (2.00–9.50) | 3.0 (2.00–14.00) | 2.00 (2.00–5.00) | 0.688c |
| Hospital stay (day), median (IQR) | 29.0 (15.00–43.50) | 30.0 (15.00–41.00) | 20.0 (15.00–61.00) | 0.947c |
| Postoperative complication, No. (%) | ||||
| 30-day mortality | 3 (13.6) | 3 (27.3) | 0 | 0.214c |
| Morbidity | 11 (50.0) | 6 (54.5) | 5 (45.5) | 0.670b |
| Pneumonia | 12 (54.5) | 6 (54.5) | 6 (54.5) | >0.999c |
| Prolonged ventilation | 1 (4.5) | 0 | 1 (9.1) | >0.999c |
| Re-admission to ICU | 7 (31.8) | 3 (27.3) | 4 (36.4) | >0.999c |
| SSI | 1 (4.5) | 1 (9.1) | 0 | >0.999c |
| Change in strategy, No. (%) | ||||
| Interruption and change of feeding route | - | 0 | 7 (63.7) | 0.001b |
| Characteristic | All (n=22) | PN (n=11) | EN (n=11) | P-valuea |
|---|---|---|---|---|
| Calorie and protein targets in ICU | ||||
| Calorie intake (kcal/kg), mean±SD | ||||
| D1 | 29.9±18.70 | 33.5±15.53 | 26.2±21.56 | 0.379 |
| D2 | 41.9±13.00 | 45.7±9.34 | 38.1±15.35 | 0.174 |
| D3 | 54.0±12.97 | 56.6±14.10 | 51.5±11.82 | 0.362 |
| D5 | 77.9±17.08 | 82.1±12.79 | 73.7±20.27 | 0.261 |
| D7 | 92.8±11.35 | 93.1±8.86 | 92.4±14.10 | 0.893 |
| Total protein (g/kg), mean±SD | ||||
| D1 | 0.5±0.35 | 0.6±0.38 | 0.4±0.37 | 0.121 |
| D2 | 0.7±0.31 | 0.9±0.70 | 0.5±0.25 | 0.001 |
| D3 | 0.9±0.31 | 1.0±0.27 | 0.7±0.26 | 0.008 |
| D5 | 1.1±0.29 | 1.3±0.28 | 1.0±0.24 | 0.034 |
| D7 | 1.3±0.21 | 1.4±0.20 | 1.2±0.18 | 0.036 |
| Biochemical parameters | ||||
| Albumin (g/L), mean±SD | ||||
| D1 | 29.2±4.03 | 28.4±4.50 | 30.1±3.51 | 0.327 |
| D3 | 27.0±3.48 | 25.5±3.01 | 28.4±3.47 | 0.055 |
| D5 | 28.5±3.02 | 28.1±2.17 | 29.0±3.74 | 0.494 |
| D7 | 28.9±5.31 | 27.3±3.90 | 30.5±6.17 | 0.153 |
| CRP (mg/L), mean±SD | ||||
| D1 | 102.3±59.37 | 121.5±70.2 | 83.2±40.66 | 0.134 |
| D3 | 200.0±102.22 | 221.7±108.40 | 178.3±95.67 | 0.331 |
| D5 | 195.3±102.48 | 225.2±66.61 | 165.4±125.09 | 0.182 |
| D7 | 163.0±98.52 | 182.0±70.83 | 144.0±120.70 | 0.380 |
| Fluid balance (mL), median (IQR) | ||||
| D1 | 1,438 (854.3 to 1,961) | 997.0 (566.0 to1,948) | 1,599 (1,200 to 2,000) | 0.158 |
| D3 | 439.5 (–218.0 to 798.5) | 292.0 (–199.0 to 576.0) | 742.0 (–674.0 to 830.0) | 0.577 |
| D5 | 354.0 (–56.0 to 605.5) | 580.0 (–8.0 to 900.0) | 67.0 (–74.0 to 400.0) | 0.061 |
| D7 | 338.0 (–30.3 to 761.5) | 520.0 (194.0 to 859.0) | 190.0 (–46.0 to 729.0) | 0.375b |
| Grade | No. (%) | P-value | ||
|---|---|---|---|---|
| All (n=22) | PN (n=11) | EN (n=11) | ||
| 0 | 8 (36.4) | 4 (36.4) | 4 (36.4) | 0.574 |
| I | 0 | 0 | 0 | |
| II | 3 (13.7) | 1 (9.1) | 2 (18.2) | |
| IIIa | 1 (4.5) | 0 | 1 (9.1) | |
| IIIb | 8 (36.4) | 5 (45.4) | 3 (27.2) | |
| IVb | 1 (4.5) | 0 | 1 (9.1) | |
| V | 1 (4.5) | 1 (9.1) | 0 | |
| Biochemical parameter | Mean±SD | t-test (df) | P-valuea | |
|---|---|---|---|---|
| Non-pneumonia (n=10) | Pneumonia (n=12) | |||
| Albumin (g/L) | 27.8±2.62 | 29.2±3.30 | –1.06 (20) | 0.302 |
| CRP (mg/L) | 137.4±96.58 | 243.5±82.69 | –2.77 (20) | 0.012 |
| Correlation | Correlation coefficient | P-valuea |
|---|---|---|
| LOS vs. albumin | –0.256 | 0.250 |
| LOS vs. CRP | 0.424 | 0.049 |
| Ventilation days vs. albumin | –0.190 | 0.397 |
| Ventilation days vs. CRP | 0.276 | 0.213 |
PN, parenteral nutrition; EN, enteral nutrition; SD, standard deviation; ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group; SGA, Subjective Global Assessment; BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; SSI, surgical site infection. Independent t-test was applied, significant P-value was set at 0.05. Chi-square test was applied, significant P-value was set at 0.05. Fisher test was applied, significant P-value was set at 0.05.
PN, parenteral nutrition; EN, enteral nutrition; ICU, intensive care unit; SD, standard deviation; CRP, C-reactive protein; IQR, interquartile range. Independent t-test, significant P-value was set at 0.05. Mann Whitney U test was applied, significant P-value was set at 0.05.
PN, parenteral nutrition; EN, enteral nutrition.
CRP, C-reactive protein; SD, standard deviation; df, degree of freedom. Independent t-test was applied, significant P-value was set at 0.05.
LOS, length of stay; CRP, C-reactive protein. Pearson correlation was applied, significant P-value was set at 0.05.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN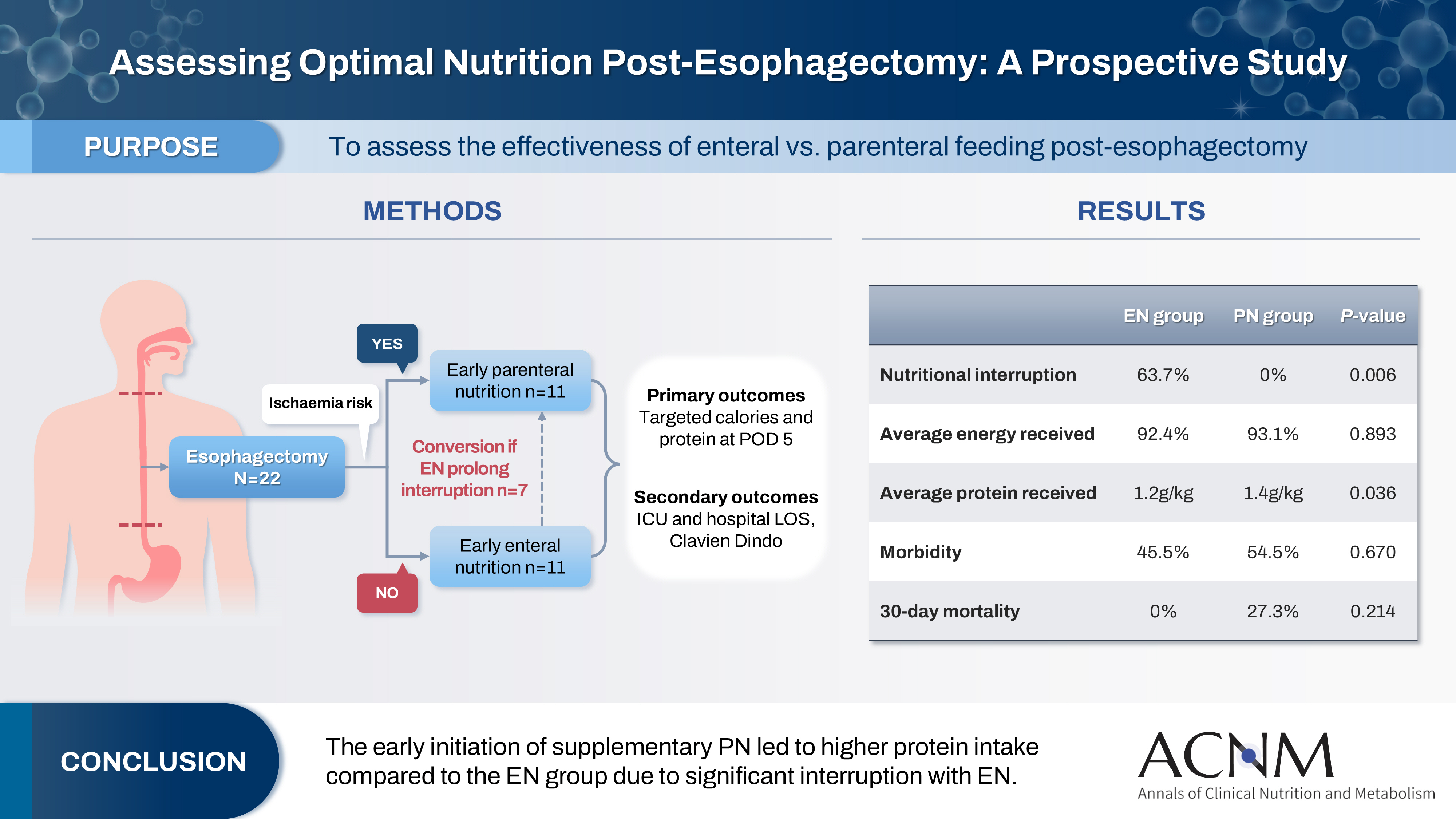
 Cite
Cite