Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 17(2); 2025 > Article
- Original Article Evaluation of the modified Nutrition Risk in Critically Ill score in Korean critically ill patients with COVID-19: a retrospective cohort study
-
Won Ho Han1,2
 , Jong-Mog Lee1,3
, Jong-Mog Lee1,3 , Jae Hoon Lee1,2
, Jae Hoon Lee1,2 , Hyun Mi Lee1
, Hyun Mi Lee1 , Ji-Yeon Kim1
, Ji-Yeon Kim1 , Mok Young Jang1
, Mok Young Jang1 , Sung-Sik Han1,4
, Sung-Sik Han1,4
-
Annals of Clinical Nutrition and Metabolism 2025;17(2):125-131.
DOI: https://doi.org/10.15747/ACNM.25.0009
Published online: August 1, 2025
1Nutrition Support Team, National Cancer Center, Goyang, Korea
2Critical Care Medicine, National Cancer Center, Goyang, Korea
3Center for Lung Cancer, National Cancer Center, Goyang, Korea
4Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, Korea
- Corresponding author: Sung-Sik Han email: sshan@ncc.re.kr
© 2025 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition · Asian Society of Surgical Metabolism and Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,778 Views
- 30 Download
Abstract
-
Purpose We evaluated the efficacy of the modified Nutrition Risk in Critically Ill (mNUTRIC) score for malnutrition screening and its association with mortality in intensive care unit (ICU) patients with COVID-19.
-
Methods The nutritional status of 129 COVID-19 ICU patients admitted between February 2021 and May 2022 was assessed using American Society for Parenteral and Enteral Nutrition/Academy of Nutrition and Dietetics (ASPEN/AND) criteria. The sensitivity, specificity, and clinical correlations of the mNUTRIC score were analyzed.
-
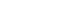
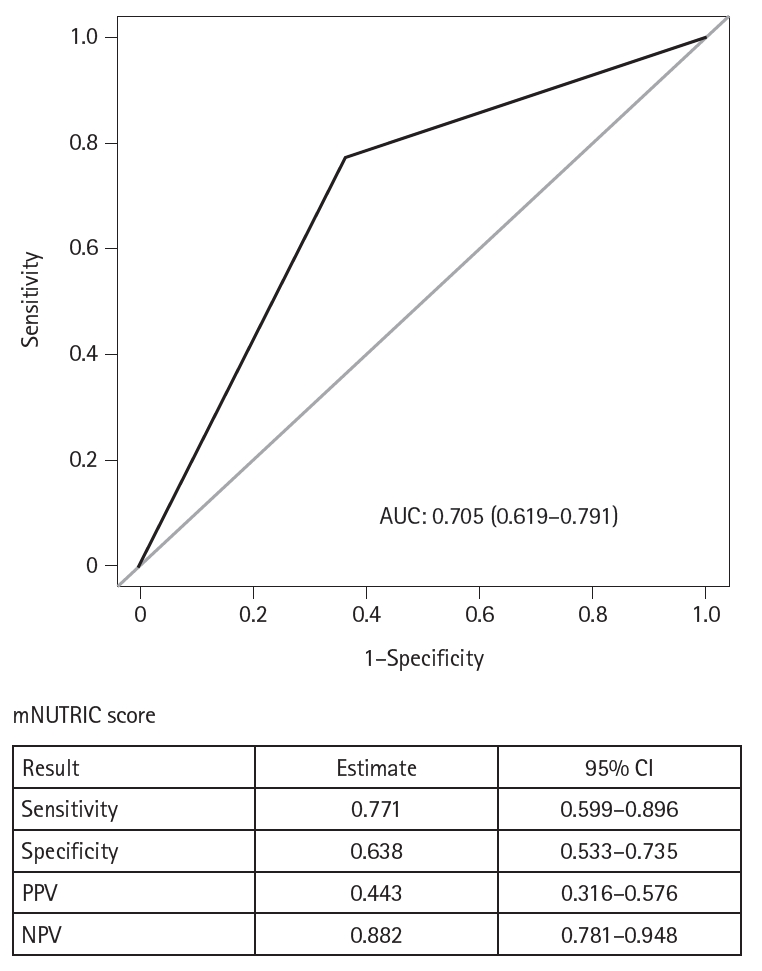
Results Of the 129 patients, 35 (27.1%) met the ASPEN/AND malnutrition criteria. Multivariable analysis identified the mNUTRIC score, underlying malignancy, and mechanical ventilation as significant factors associated with malnutrition. The mNUTRIC score had a sensitivity of 77.1% and specificity of 63.8% (area under the curve [AUC], 0.71; 95% confidence interval [CI], 0.62–0.79) for diagnosing malnutrition, improving to 88.6% and 80.9%, respectively, after adjusting for malignancy and ventilation (AUC, 0.89; 95% CI, 0.82–0.95). Patients with a low mNUTRIC score had a mortality rate of 2.9% and a median ICU stay of 7.7 days (range, 0–84.2 days), whereas those with a high score (≥5) had a mortality rate of 13.1% and a median ICU stay of 10.2 days (range, 1.4–88.5 days) (P=0.046 and P=0.011, respectively).
-
Conclusion The mNUTRIC score is an effective screening tool for malnutrition in ICU patients with COVID-19, especially those with malignancy or requiring mechanical ventilation, and is strongly associated with mortality and length of ICU stay.
Introduction
Methods
Results
Discussion
Authors’ contribution
Conceptualization: SSH. Data curation: WHH. Formal analysis: JHL. Investigation: JML, SSH. Supervision: SSH. Writing–original draft: WHH, JHL, SSH. Writing–review & editing: HML, JYK, MYJ, JML. All authors read and approved the final manuscript.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose.
Funding
This work was supported by a grant (NCC 2310350-3) from the National Cancer Center, Republic of Korea.
Data availability
Contact the corresponding author for research data availability.
Acknowledgments
None.
Supplementary materials
Values are presented as number (%), mean±SD, or median (IQR).
BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; mNUTRIC score, modified Nutrition Risk in Critically Ill score; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
aChi-square test.
bt-test.
cFisher exact test.
dWilcoxon rank sum test.
OR, odds ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; mNUTRIC score, modified Nutrition Risk in Critically Ill score; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
- 1. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. ArticlePubMed
- 2. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. ArticlePubMed
- 3. Wang PY, Li Y, Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition 2021;84:111104.ArticlePubMedPMC
- 4. Wierdsma NJ, Kruizenga HM, Konings LA, Krebbers D, Jorissen JR, Joosten MI, et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN 2021;43:369-76. ArticlePubMedPMC
- 5. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. ArticlePubMed
- 6. Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr 2004;92:799-808. ArticlePubMed
- 7. Arabi YM, Preiser JC. A critical view on primary and secondary outcome measures in nutrition trials. Intensive Care Med 2017;43:1875-7. ArticlePubMedPDF
- 8. Gelfman DM. Will the traditional physical examination be another casualty of COVID-19? Am J Med 2021;134:299-300. ArticlePubMedPMC
- 9. Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R268.ArticlePubMedPMCPDF
- 10. Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK, et al. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr 2016;35:158-62. ArticlePubMed
- 11. Mukhopadhyay A, Henry J, Ong V, Leong CS, Teh AL, van Dam RM, et al. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin Nutr 2017;36:1143-8. ArticlePubMed
- 12. de Vries MC, Koekkoek WK, Opdam MH, van Blokland D, van Zanten AR. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr 2018;72:428-35. ArticlePubMedPMCPDF
- 13. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. ArticlePubMedPDF
- 14. Hanidziar D, Bittner EA. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg 2020;131:e40-1. ArticlePubMedPMC
- 15. Flinspach AN, Booke H, Zacharowski K, Balaban U, Herrmann E, Adam EH, et al. High sedation needs of critically ill COVID-19 ARDS patients: a monocentric observational study. PLoS One 2021;16:e0253778.ArticlePubMedPMC
- 16. Kundu R, Seeger R, Elfassy MD, Rozenberg D, Ahluwalia N, Detsky ME, et al. The association between nutritional risk index and ICU outcomes across hematologic malignancy patients with acute respiratory failure. Ann Hematol 2023;102:439-45. ArticlePDF
- 17. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I, et al. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch 2014;68:263-7. ArticlePubMedPMC
- 18. Henry L. Effect of malnutrition on cancer patients. In: Shaw C, ed. Nutrition and cancer. Blackwell Publishing Ltd.; 2010. p. 45-82.
- 19. Berardi G, Antonelli G, Colasanti M, Meniconi R, Guglielmo N, Laurenzi A, et al. Association of sarcopenia and body composition with short-term outcomes after liver resection for malignant tumors. JAMA Surg 2020;155:e203336.ArticlePubMedPMC
- 20. Wang H, Yang R, Xu J, Fang K, Abdelrahim M, Chang L, et al. Sarcopenia as a predictor of postoperative risk of complications, mortality and length of stay following gastrointestinal oncological surgery. Ann R Coll Surg Engl 2021;103:630-7. ArticlePubMedPMC
- 21. Fogarty MJ, Mantilla CB, Sieck GC. Impact of sarcopenia on diaphragm muscle fatigue. Exp Physiol 2019;104:1090-9. ArticlePubMedPMCPDF
- 22. Woo HY, Oh SY, Lee H, Ryu HG. Evaluation of the association between decreased skeletal muscle mass and extubation failure after long-term mechanical ventilation. Clin Nutr 2020;39:2764-70. ArticlePubMed
References
Figure & Data
REFERENCES
Citations

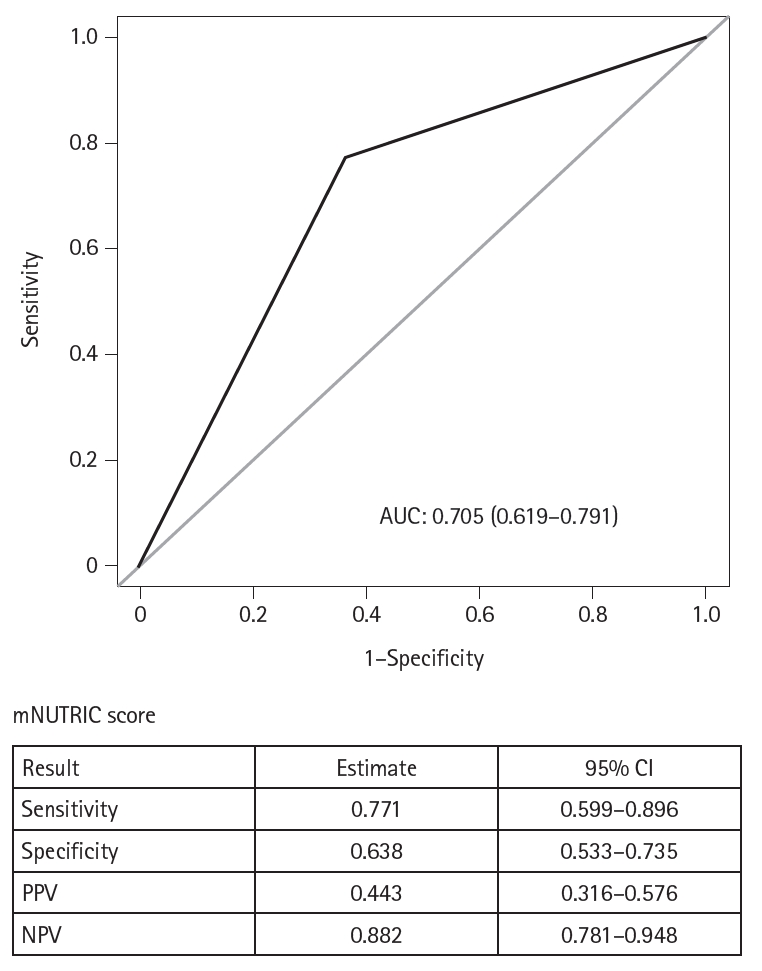
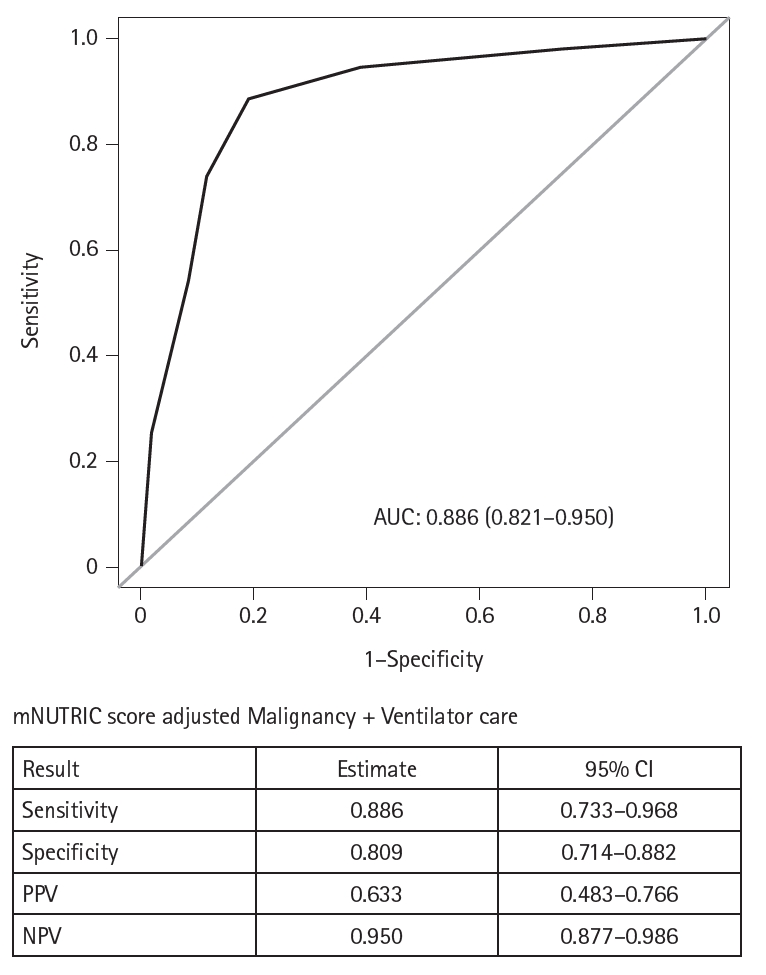
Fig. 1.
Fig. 2.
| Variables | No malnutrition (n=94) | Malnutrition (n=35) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 52 (55.3) | 17 (48.6) | 0.494a |
| Female | 42 (44.7) | 18 (51.4) | |
| Age (yr) | 63.0±13.0 | 67.7±11.8 | 0.066b |
| BMI (kg/m2) | 25.0±4.2 | 22.8±3.9 | 0.006b |
| Underlying disease | |||
| DM | 23 (24.5) | 14 (40.0) | 0.082a |
| HTN | 46 (48.9) | 20 (57.1) | 0.407a |
| COPD | 5 (5.3) | 1 (2.9) | >0.999c |
| CVA | 12 (12.8) | 6 (17.1) | 0.571c |
| Malignancy | 23 (24.5) | 18 (51.4) | 0.003a |
| Nutrition route | <0.001a | ||
| No nutritional support | 62 (66.0) | 2 (5.7) | |
| Enteral feeding | 12 (12.8) | 24 (68.6) | |
| Parenteral | 20 (21.3) | 9 (25.7) | |
| APACHE II | 25 (12–34) | 27 (13–50) | 0.006d |
| SOFA | 1 (0–7) | 2 (0–10) | 0.002d |
| mNUTRIC score | 4 (1–7) | 6 (3–8) | <0.001d |
| Initial laboratory tests | |||
| Albumin (g/dL) | 3.3±0.6 | 3.0±0.4 | 0.005b |
| CRP (mg/dL) | 8.2±6.0 | 11.8±7.7 | 0.008b |
| Ventilator care | 15 (16.0) | 24 (68.6) | <0.001a |
| CRRT | 2 (2.1) | 8 (22.9) | <0.001c |
| ECMO | 4 (4.3) | 4 (11.4) | 0.211c |
| ICU length of stay (day) | 7.5 (2.0–84.2) | 13.0 (2.4–88.5) | <0.001d |
| Outcome | 0.004c | ||
| Survival | 91 (96.8) | 28 (80.0) | |
| Mortality | 3 (3.2) | 7 (20.0) |
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Sex | Male | Ref | |||
| Female | 1.31 (0.60–2.85) | 0.495 | |||
| Age | 1.03 (0.99–1.06) | 0.069 | |||
| BMI | 0.87 (0.79–0.96) | 0.009 | |||
| Underlying disease | |||||
| DM | No | Ref | |||
| Yes | 2.05 (0.90–4.69) | 0.086 | |||
| HTN | No | Ref | |||
| Yes | 1.39 (0.63–3.04) | 0.408 | |||
| COPD | No | Ref | |||
| Yes | 0.52 (0.05–4.64) | 0.561 | |||
| CVA | No | Ref | |||
| Yes | 1.41 (0.48–4.11) | 0.525 | |||
| Malignancy | No | Ref | Ref | ||
| Yes | 3.26 (1.45–7.36) | 0.004 | 7.55 (2.21–25.77) | 0.001 | |
| APACHE II | 1.13 (1.04–1.23) | 0.003 | |||
| SOFA | 1.52 (1.19–1.94) | 0.001 | |||
| mNUTRIC score | Low | Ref | Ref | ||
| High | 5.95 (2.43–14.56) | <0.001 | 3.88 (1.39–10.82) | 0.010 | |
| Initial laboratory tests | |||||
| Albumin | 0.34 (0.14–0.86) | 0.023 | |||
| CRP | 1.08 (1.01–1.14) | 0.010 | |||
| Ventilator care | No | Ref | Ref | ||
| Yes | 11.48 (4.66–28.32) | <0.001 | 16.43 (4.93–54.72) | <0.001 | |
| CRRT | No | Ref | |||
| Yes | 13.62 (2.73–68.00) | 0.002 | |||
| ECMO | No | Ref | |||
| Yes | 2.90 (0.68–12.31) | 0.148 | |||
| ICU length of stay | 1.04 (1.01–1.07) | 0.004 | |||
| Variable | Low mNUTRIC score (<5) | High mNUTRIC score (≥5) | P-value |
|---|---|---|---|
| Mortality | 2 (2.9) | 8 (13.1) | 0.046a |
| ICU length of stay | 7.7 (0–84.2) | 10.2 (1.4–88.5) | 0.011b |
| Ventilator care | 12 (17.6) | 27 (44.3) | 0.001a |
| ECMO | 3 (4.4) | 5 (8.2) | 0.475c |
Values are presented as number (%), mean±SD, or median (IQR). BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; mNUTRIC score, modified Nutrition Risk in Critically Ill score; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range. aChi-square test. bt-test. cFisher exact test. dWilcoxon rank sum test.
OR, odds ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; mNUTRIC score, modified Nutrition Risk in Critically Ill score; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Values are presented as number (%) or median (range). mNUTRIC score, modified Nutrition Risk in Critically Ill score; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation. aChi-square test. bWilcoxon rank sum test. cFisher exact test.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite