Scopus, KCI, KoreaMed

Articles
- Page Path
- HOME > Ann Clin Nutr Metab > Volume 17(2); 2025 > Article
- Original Article Peripheral vein infusions of amino acids prevent early postoperative weight loss after robot-assisted radical transmediastinal esophagectomy: a retrospective study in Japan
-
Tomonori Narita1,2
 , Kazuhiko Fukatsu2,3
, Kazuhiko Fukatsu2,3 , Satoshi Murakoshi2,3,4
, Satoshi Murakoshi2,3,4 , Reo Inoue3
, Reo Inoue3 , Kenichi Kono3
, Kenichi Kono3 , Midori Noguchi3
, Midori Noguchi3 , Nana Matsumoto2
, Nana Matsumoto2 , Shoh Yajima1
, Shoh Yajima1 , Koichi Yagi1
, Koichi Yagi1 , Yoshifumi Baba1
, Yoshifumi Baba1
-
Annals of Clinical Nutrition and Metabolism 2025;17(2):149-155.
DOI: https://doi.org/10.15747/ACNM.25.0012
Published online: August 1, 2025
1Department of Gastrointestinal Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
2Operating Room Management and Surgical Metabolism, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
3Surgical Center, The University of Tokyo Hospital, Tokyo, Japan
4Faculty of Health and Social Work, School of Nutrition and Dietetics, The Kanagawa University of Human Services, Kanagawa, Japan
- Corresponding author: Kazuhiko Fukatsu email: fukatsu-1su@h.u-tokyo.ac.jp
© 2025 The Korean Society of Surgical Metabolism and Nutrition · The Korean Society for Parenteral and Enteral Nutrition · Asian Society of Surgical Metabolism and Nutrition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,169 Views
- 17 Download
Abstract
-
Purpose Postoperative body weight loss (PBWL) is linked to poor long-term outcomes following esophagectomy for esophageal cancer, making perioperative nutrition critically important. Although minimally invasive procedures such as robot-assisted radical transmediastinal esophagectomy (RA-TME) have become more prevalent, less attention has been paid to perioperative nutritional management. This study evaluates the impact of intravenous (IV) amino acid infusions on PBWL in patients undergoing RA-TME.
-
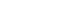
Methods We retrospectively analyzed 155 patients who underwent RA-TME for esophageal or esophagogastric junction cancer at our hospital between 2011 and 2022. Patients were divided into two groups: AA(+) (n=73, received IV amino acids between postoperative days 1–6) and AA(–) (n=82, did not receive IV amino acids). Oral or enteral nutrition was withheld until postoperative day 6. We compared nutrient intake, postoperative outcomes, and nutritional status between groups.
-
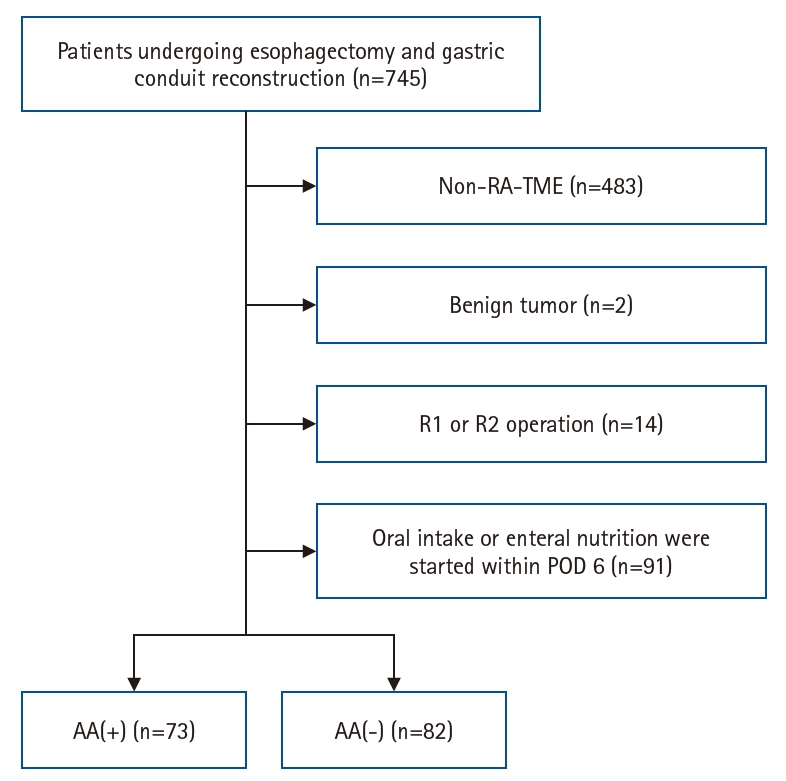
Results Patient backgrounds, surgical outcomes, and complication rates were similar in both groups. However, the AA(+) group received significantly greater energy and nutrient intake. PBWL at 2 weeks post-surgery was significantly lower in the AA(+) group than in the AA(–) group (6.50% vs. 8.15%, P=0.0091).
-
Conclusion IV amino acid infusion may help mitigate early PBWL after RA-TME.
Graphical abstract
Introduction
Methods
Results
Discussion
Authors’ contribution
Conceptualization: TN, KF, SM, RI, KK, MN, NM. Data curation: TN. Formal analysis: TN. Investigation: TN. Methodology: TN. Project administration: TN. Resources: TN. Software: TN. Supervision: KF. Validation: TN, KF, IR, KK. Visualization: TN. Writing–original draft: TN. Writing–review & editing: TN, KF, SM, RI, KK, MN, NM, SY, KY, YB. All authors read and approved the final manuscript.
Conflict of interest
Contact the corresponding author for research data availability.
Funding
None.
Data availability
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/G6M5ZS
Acknowledgments
None.
Supplementary materials
Values are presented as number (%) or median (interquartile range).
AA(+), intravenous amino acids administered by postoperative day 6; AA(–), no amino acids administered; BMI, body mass index; SPPB, Short Physical Performance Battery; SMI, skeletal muscle index; ASA-PS, American Society of Anesthesiologists physical status; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet to lymphocyte ratio; PNI, prognostic nutritional index.
aSPPB scores include missing values.
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49. ArticlePubMedPDF
- 2. Koterazawa Y, Oshikiri T, Takiguchi G, Urakawa N, Hasegawa H, Yamamoto M, et al. Severe weight loss after minimally invasive oesophagectomy is associated with poor survival in patients with oesophageal cancer at 5 years. BMC Gastroenterol 2020;20:407.ArticlePubMedPMCPDF
- 3. Kubo Y, Miyata H, Sugimura K, Shinno N, Asukai K, Hasegawa S, et al. Prognostic implication of postoperative weight loss after esophagectomy for esophageal squamous cell cancer. Ann Surg Oncol 2021;28:184-93. ArticlePubMedPDF
- 4. Yamamoto K, Tanaka K, Yamasaki M, Yamashita K, Makino T, Saito T, et al. Early postoperative weight loss is associated with poor prognosis in patients with esophageal cancer. Esophagus 2022;19:596-603. ArticlePubMedPDF
- 5. Mori K, Yamagata Y, Aikou S, Nishida M, Kiyokawa T, Yagi K, et al. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus 2016;29:429-34. ArticlePubMedPMCPDF
- 6. Yoshimura S, Mori K, Yamagata Y, Aikou S, Yagi K, Nishida M, et al. Quality of life after robot-assisted transmediastinal radical surgery for esophageal cancer. Surg Endosc 2018;32:2249-54. ArticlePubMedPMCPDF
- 7. Mori K, Yamagata Y, Wada I, Shimizu N, Nomura S, Seto Y, et al. Robotic-assisted totally transhiatal lymphadenectomy in the middle mediastinum for esophageal cancer. J Robot Surg 2013;7:385-7. ArticlePubMedPMCPDF
- 8. Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. John Wiley & Sons; 2017.
- 9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. ArticlePubMedPMC
- 10. Fukatsu K. Role of nutrition in gastroenterological surgery. Ann Gastroenterol Surg 2019;3:160-8. ArticlePubMedPMCPDF
- 11. Konosu M, Iwaya T, Kimura Y, Akiyama Y, Shioi Y, Endo F, et al. Peripheral vein infusions of amino acids facilitate recovery after esophagectomy for esophageal cancer: retrospective cohort analysis. Ann Med Surg (Lond) 2017;14:29-35. ArticlePubMedPMC
References
Figure & Data
REFERENCES
Citations

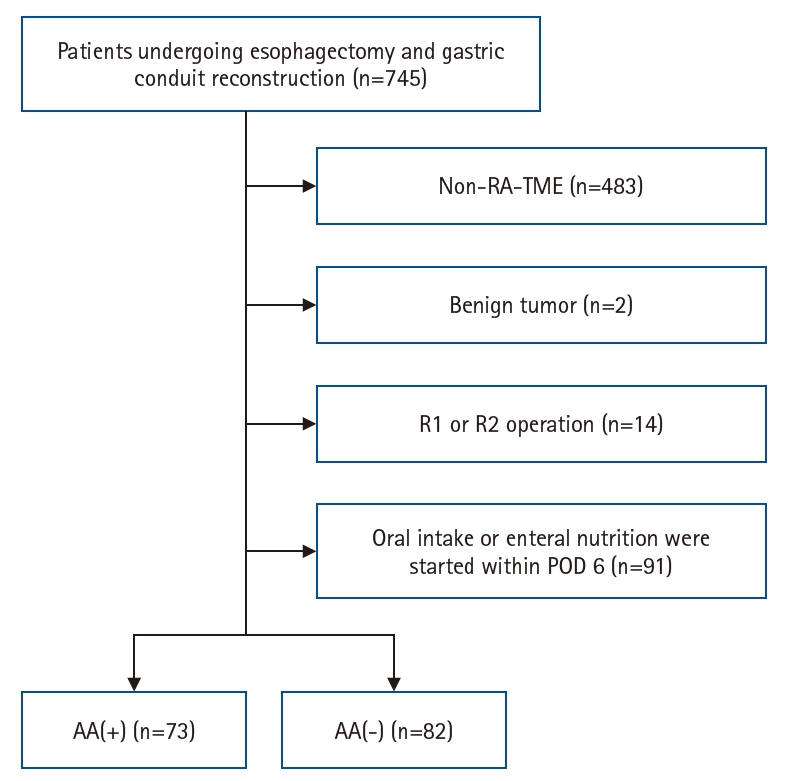
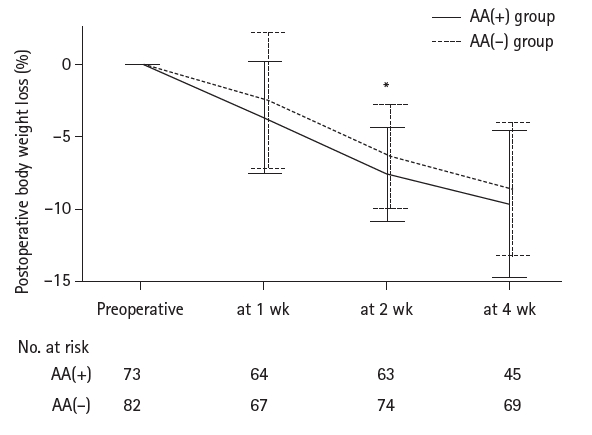
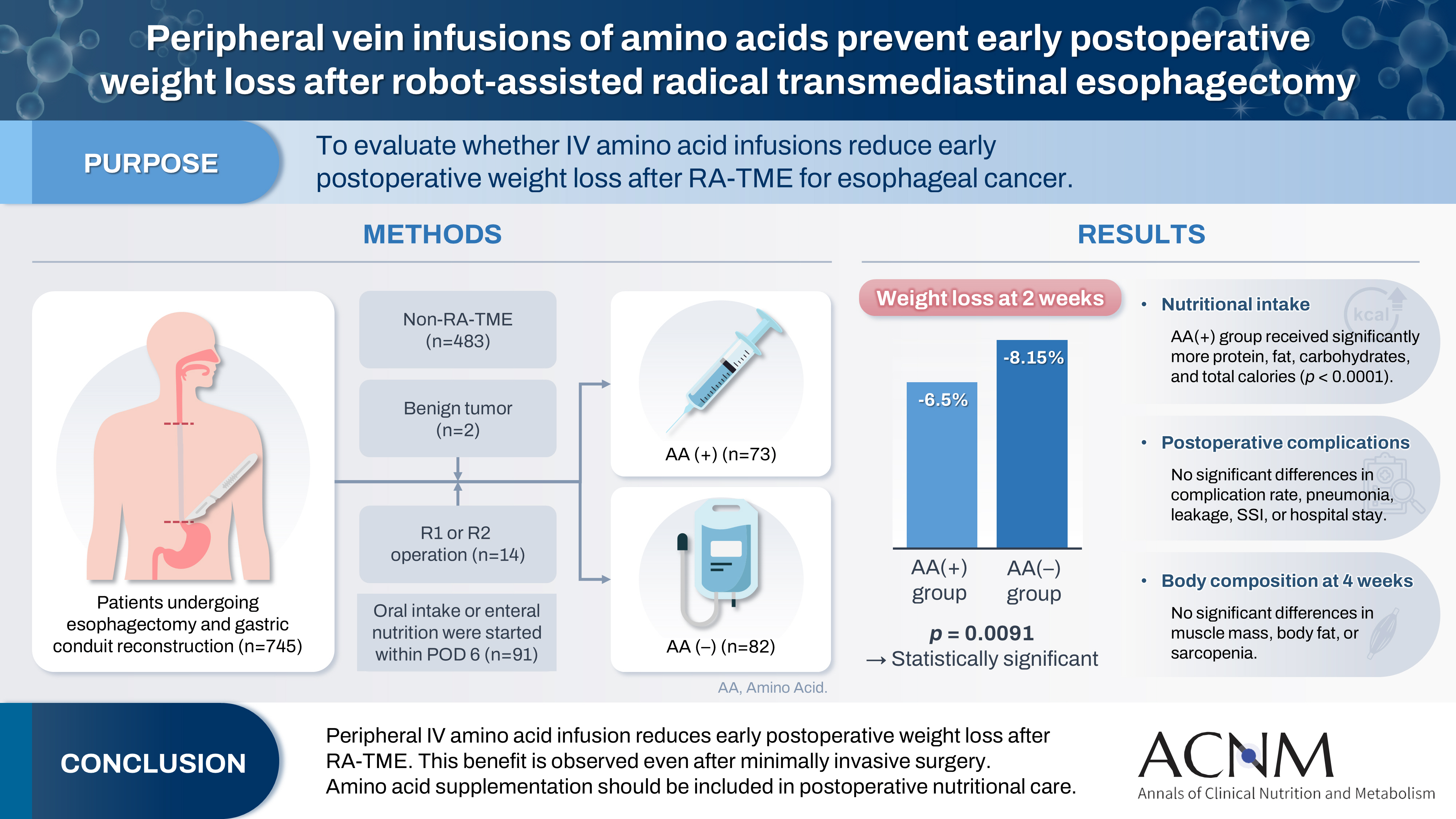
Fig. 1.
Fig. 2.
Graphical abstract
| Characteristic | AA(+) group (n=73) | AA(–) group (n=82) | P-value |
|---|---|---|---|
| Sex | 0.1516 | ||
| Male | 59 (80.82) | 73 (89.02) | |
| Female | 14 (19.18) | 9 (10.98) | |
| Age (yr) | 68 (60–74) | 68 (61–72) | 0.9143 |
| ≥75 yr | 15 (19.35) | 15 (20.55) | 0.7228 |
| Height (cm) | 165.2 (160.3–169.6) | 166.6 (162.4–171) | 0.1500 |
| Body weight (kg) | 60.3 (53.8–66.0) | 61.9 (53.6–68.8) | 0.4801 |
| BMI (kg/m2) | 21.8 (19.7–24.2) | 21.7 (20.2–23.7) | 0.7592 |
| Obese (BMI ≥25 kg/m2) | 10 (13.70) | 13 (15.85) | 0.7064 |
| Underweight (BMI ≤18.5 kg/m2) | 11 (15.07) | 10 (12.20) | 0.6018 |
| Muscle mass (kg) | 45.1 (37.1–48.1) | 46.5 (42.1–49.9) | 0.1016 |
| Body fat percentage (%) | 16.7 (14.7–22.6) | 20.0 (16.1–24.1) | 0.0940 |
| SPPB score (point) | 12 (12–12) | 12 (11–12) | 0.2642 |
| SPPB scorea | 0.9064 | ||
| ≤9 points | 2 (4.00) | 2 (2.70) | |
| ≥10 points | 48 (96.00) | 72 (97.30) | |
| SMI (kg/m2) | 7.15 (6.05–7.78) | 7.50 (6.80–8.10) | 0.0447 |
| Low SMI | 10 (35.71) | 22 (22.00) | 0.1385 |
| Sarcopenia | 7 (25.00) | 13 (13.00) | 0.1222 |
| Preoperative chemotherapy | 34 (46.58) | 46 (56.10) | 0.2364 |
| ASA-PS classification | 0.3710 | ||
| 1 or 2 | 68 (93.15) | 73 (89.02) | |
| ≥3 | 5 (6.85) | 9 (10.98) | |
| White blood cells, (/μL) | 6,500 (5,250–8,050) | 6,200 (4,900–7,625) | 0.2869 |
| Neutrophils (/μL) | 4,340 (3,247–5,954) | 4,085 (3,196–5,104) | 0.0713 |
| Lymphocytes (/μL) | 1,342 (1,129–1,584) | 1,488 (1,223–1,773) | 0.1440 |
| Hemoglobin (g/dL) | 12.4 (10.3–13.7) | 12.0 (10.7–13.8) | 0.8269 |
| Platelets (×104/μL) | 25.2 (21.6–29.7) | 24.7 (19.4–30.5) | 0.5554 |
| Serum albumin (mg/dL) | 3.9 (3.7–4.2) | 3.9 (3.6–4.2) | 0.7749 |
| AST (U/L) | 19.0 (16.0–22.0) | 20.0 (16.0–22.3) | 0.7531 |
| ALT (U/L) | 14.0 (11.0–17.0) | 13.0 (10.0–19.3) | 0.9871 |
| γ-GTP (U/L) | 29 (21–41) | 26 (20–37) | 0.3431 |
| Total bilirubin (mg/dL) | 0.5 (0.4–0.7) | 0.6 (0.4–0.7) | 0.5849 |
| CRP (mg/dL) | 0.07 (0.03–0.25) | 0.07 (0.04–0.19) | 0.9141 |
| NLR | 3.1 (2.4–4.8) | 2.7 (2.1–4.0) | 0.0161 |
| PLR | 195.2 (144.7–254.4) | 155.0 (126.1–235.5) | 0.0987 |
| PNI | 46.4 (43.5–48.8) | 47.0 (43.4–49.0) | 0.5164 |
| AA(+) group (n=73) | AA(–) group (n=82) | P-value | |
|---|---|---|---|
| Operative time (min) | 429.0 (372.0–478.5) | 430.5 (381.5–478.3) | 0.7348 |
| Intraoperative blood loss (mL) | 180.0 (80.0–290.0) | 180.0 (87.5–386.3) | 0.4568 |
| Lymphadenectomy | 0.0013 | ||
| Three-field | 51 (69.86) | 74 (90.24) | |
| Two-field | 22 (30.14) | 8 (9.76) | |
| Reconstruction route | 0.5858 | ||
| Posterior mediastinal route | 69 (84.52) | 79 (96.34) | |
| Non-posterior mediastinal route | 4 (5.48) | 3 (3.66) |
| AA(+) group (n=73) | AA(–) group (n=82) | P-value | |
|---|---|---|---|
| Amounts on POD 6 | |||
| Protein and AA (g) | 45.0 (45.0–60.0) | 0 (0–0) | <0.0001 |
| Fat (g) | 0 (0–21.2) | 0 (0–0) | <0.0001 |
| Carbohydrates (g) | 150.0 (112.5–152.2) | 86.0 (64.5–86.0) | <0.0001 |
| Energy (kcal) | 840 (630–1,040) | 344 (258–344) | <0.0001 |
| Total amounts for POD 1-6 | |||
| Protein and AA (g) | 150.0 (97.5–195.0) | 0 (0–0) | <0.0001 |
| Fat (g) | 0 (0–63.6) | 0 (0–0) | <0.0001 |
| Carbohydrates (g) | 643.5 (577.5–775.9) | 521.3 (448.3–545.4) | <0.0001 |
| Energy (kcal) | 3,350 (2,898–4,336) | 2,092 (1,824–2,210) | <0.0001 |
| PN (mL) | 13,620 (12,370–14,720) | 13,250 (12,075–14,590) | 0.4890 |
| Total amounts for POD 1-14 | |||
| Protein and AA (g) | 561.0 (488.8–642.1) | 250.4 (184.6–336.2) | <0.0001 |
| Fat (g) | 163.0 (105.9–251.1) | 107.8 (72.4–131.7) | <0.0001 |
| Carbohydrates (g) | 1,966 (1,742–2,221) | 1,647 (1,457–1,803) | <0.0001 |
| Energy (kcal) | 12,151 (10,371–12,889) | 8,436 (7,605–9,686) | <0.0001 |
| PN (mL) | 23,680 (19,880–27,535) | 22,325 (19,163–27,363) | 0.1272 |
| AA(+) group (n=73) | AA(–) group (n=82) | P-value | |
|---|---|---|---|
| Postoperative complications with CD ≥2 | 41 (56.16) | 47 (57.32) | 0.885 |
| Postoperative complications with CD ≥3 | 24 (32.88) | 17 (20.73) | 0.0871 |
| Pneumonia | 14 (19.18) | 11 (13.41) | 0.3302 |
| Anastomotic leakage | 11 (15.07) | 16 (19.51) | 0.4666 |
| Superficial surgical site infection | 4 (5.48) | 5 (6.10) | 0.8695 |
| Postoperative hospitalization (day) | 21 (17–31) | 18 (17–26) | 0.4564 |
| Pathological T factor | 0.0722 | ||
| pT1 or 2 | 56 (76.71) | 52 (63.41) | |
| pT3 or 4 | 17 (23.29) | 30 (36.59) | |
| Lymph node metastasis | 0.4776 | ||
| Presence | 38 (52.05) | 38 (46.34) | |
| Absence | 35 (47.95) | 44 (53.66) |
| AA(+) group (n=45) | AA(–) group (n=69) | P-value | |
|---|---|---|---|
| Muscle mass (kg) | 42.6 (35.7–46.7) | 43.9 (39.9–46.8) | 0.2943 |
| Muscle mass loss (kg) | 1.6 (0.7–3.4) | 2.8 (1.5–4.4) | 0.1148 |
| Muscle mass loss change (%) | 4.4 (1.8–7.6) | 5.9 (3.2–9.3) | 0.1893 |
| Body fat percentage (%) | 18.5 (14.7–20.8) | 16.1 (13.4–20.1) | 0.1823 |
| Body fat percentage change (%) | 2.9 (1.1–3.6) | 2.0 (1.1–3.4) | 0.4283 |
| SPPB score (point) | 12 (11–12) | 12 (11–12) | 0.7922 |
| Low SPPB scorea | 0.1676 | ||
| ≤9 points | 5 (12.50) | 3 (4.92) | |
| ≥10 points | 35 (87.50) | 58 (95.08) | |
| SMI (kg/m2) | 6.7 (6.0–7.2) | 7.0 (6.0–7.0) | 0.6764 |
| Low SMI | 26 (57.78) | 35 (50.72) | 0.3843 |
| Sarcopenia | 19 (42.22) | 23 (33.33) | 0.3362 |
Values are presented as number (%) or median (interquartile range). AA(+), intravenous amino acids administered by postoperative day 6; AA(–), no amino acids administered; BMI, body mass index; SPPB, Short Physical Performance Battery; SMI, skeletal muscle index; ASA-PS, American Society of Anesthesiologists physical status; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet to lymphocyte ratio; PNI, prognostic nutritional index. aSPPB scores include missing values.
Values are presented as median (interquartile range) or number (%). AA(+), intravenous amino acids administered by postoperative day 6; AA(–), no amino acids administered.
Values are presented as median (interquartile range). AA(+), intravenous amino acids administered by POD 6; AA(–), no amino acids administered; POD, postoperative day; AA, amino acid; PN, parenteral nutrition.
Values are presented as number (%) or median (interquartile range). AA(+), intravenous amino acids administered by postoperative day 6; AA(–), no amino acids administered; CD, Clavien-Dindo classification.
Values are presented as median (interquartile range) or number (%). AA(+), intravenous amino acids administered by postoperative day 6; AA(–), no amino acids administered; SPPB, Short Physical Performance Battery; SMI, skeletal muscle index. aSPPB scores include missing values.

 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN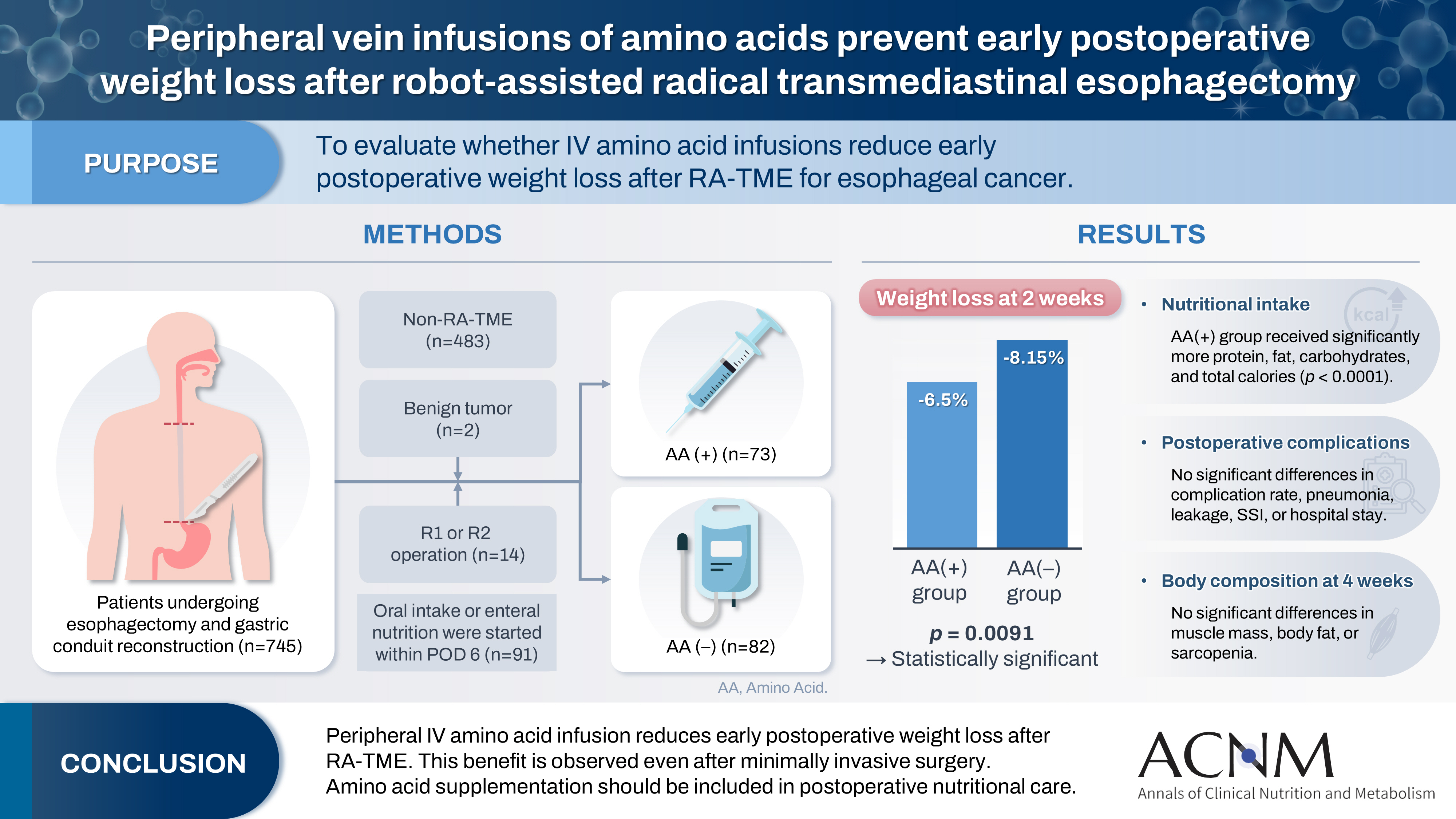
 Cite
Cite