Abstract
-
Purpose
The present study uses healthy human volunteers to examine the insulinotropic action of L-carnitine and branched-chain amino acids (BCAAs) after energy intake.
-
Methods
A total of 39 young, healthy human volunteers were assigned to receive oral doses of either L-carnitine alone (L group, n=10) or L-carnitine combined with a single or long-term continuous dose of BCAAs. Controls (C group, n=16) received none of these. L-carnitine was administered orally at 1,000 mg/day for 14 days, and BCAA was administered orally either once just before exercise (L+SB group, n=6), or every day for 14 days (L+CB group, n=7) until 2 days before the experiment. After overnight fasting, 200 kcal of glucose and oral nutritional supplement were administered to prevent hypoglycemia. Blood glucose, free-fatty acid, and serum insulin levels were measured to examine the insulinotropic action before and after exercise.
-
Results
Blood glucose and serum insulin levels in the L group were significantly lower than those in the C group. While the serum insulin levels were higher after energy administration than those in the fasting state in all groups, these were significantly higher in the L+SB group and in the L+CB group compared with those in the L group. The insulinotropic action after energy intake remained even after the repeated administration of BCAA discontinued 2 days before the experiment and even after serum BCAA levels remained the same.
-
Conclusion
While the insulinotropic action appeared after a single dose of BCAA, it was also potentiated by long-term repeated oral administration of BCAA.
-
Keywords: Blood glucose; Branched-chain amino acids; Carnitine; Exercise; Insulins
Introduction
Background
Nutritional therapy using various nutrients is expected to prevent sarcopenia and type 2 diabetes, which occur at a high rate in the elderly [
1-
5]. Administration of amino acids, and branched-chain amino acids (BCAAs) in particular, have been reported to have important nutritional effects in preventing skeletal muscle loss and improving blood glucose regulation by overcoming anabolic resistance associated with aging [
6,
7]. These metabolic effects of BCAAs can be attributed not only to their anabolic effects as nutrients, but also to their potent insulinotropic action (ITA) [
8]. Studies have suggested that ITA induced by administration of amino acids exerts its effects by directly promoting insulin secretion and promoting the exocytosis of intracellular insulin granules and serving as a substrate for increasing the ratio of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) [
9]. However, it is not clear whether the actual anabolic effect of amino acid administration on human skeletal muscle is a single effect of amino acid administration itself or an effect of ITA induced by amino acid administration.
L-carnitine (LCAR) promotes lipid utilization (oxidation), exceeding carbohydrate oxidation by activating the tricarboxylic acid (TCA) cycle and resulting in the accumulation of the intermediate metabolite acylcarnitine, which can reduce insulin sensitivity. Acylcarnitines present
in vivo are reportedly associated with insulin resistance in humans [
10-
14].
In this study, we investigated the effects of LCAR and BCAA on ITA after energy administration before and after exercise-induced stress in healthy young women who received long-term continuous administration of LCAR and BCAA.
Methods
Ethics statement
This clinical study was conducted after the protocol received the approval from the Institutional Review Board of Wayo Women’s University (Approval No. 1415). Before the start of this clinical trial, the research was fully explained and the written informed consent of participating the study was obtained from all the subjects.
Study design
Setting
This study was performed at the Department of Ecology, Wayo Women’s University from April 2015 to March 2018. The recruitment of healthy individuals was obtained from the students of the Department of Ecology, Wayo Women’s University, and was not obtained by the public announcement.
Participants
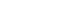
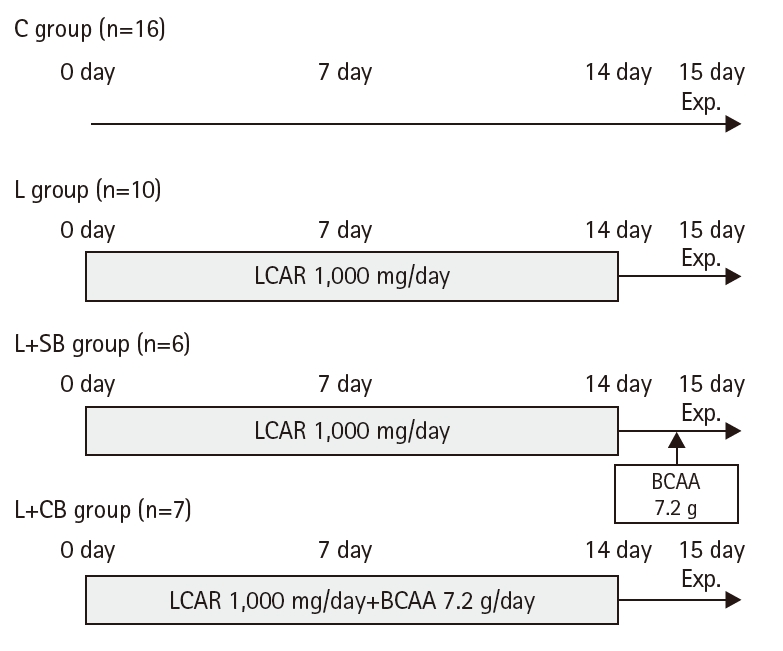
Thirty-nine subjects (mean age of 20.8±0.1 years) were included in group C (n=16), who received neither LCAR nor BCAA, and the LCAR-alone group (L), who received LCAR only. In group L, LCAR was administered at 1,000 mg/day for 14 days (
Fig. 1).
Members of the L+SB (L-carnitine + single dose of BCAA) group received LCAR for 14 days up to 2 days before the clinical trial and a single dose of BCAA on the day of the clinical trial, while the L+SB group received LCAR for 14 days up to 2 days before the clinical trial and a single BCAA dose on the day of the clinical trial. In the L+CB (L-carnitine + continuous dose of BCAA) group, LCAR (1,000 mg/day) and BCAA (7.2 g/day) were both administered orally for 14 days up to 2 days before the clinical trial. The background of the subjects in each group is shown in
Table 1.
The LCAR (L-carnitine–containing processed food; Carnipure) was provided by Lonza Japan K.K. in capsules (250 mg/capsule), and two capsules were administered with water before breakfast and dinner (
Fig. 1). The composition of Carnipure is as follows: each capsule, weighing 0.576 g, contains 250 mg of L-carnitine and 1.29 kcal of energy, 0.42 g of protein, 0.00 g of fat, 0.12 g of carbohydrate, and 0.09 mg of sodium.
Subjects in the L+SB group received LCAR at 1,000 mg/day orally every day for 14 days up to 1 day before the day of the clinical study, and 7.2 g of BCAA on the day of the clinical study 2 hours before the exercise.
Subjects in the L+CB group received 1,000 mg/day of LCAR orally plus 3 packs of amino acid–containing food, Amino Vital Gold (Ajinomoto Co., Inc.), containing 7.2 g of BCAA daily for 14 days up to 2 days before the day of the clinical study. The amino acid composition of the amino acid–containing food used in this clinical study included nine essential amino acids (EAAs), of which BCAA (L-leucine, L-isoleucine, and L-valine) accounted for 62% (by weight) [
15,
16]. The L+SB and L+CB groups increased the LCAR and BCAA pool size in the body. In the L+SB and L+CB groups, both LCAR and BCAA were terminated 2 days before the clinical trial to ensure that neither serum LCAR nor BCAA levels were elevated on the day of the clinical trial. All experimental groups were instructed in advance to follow a normal diet as much as possible without any dietary restrictions.
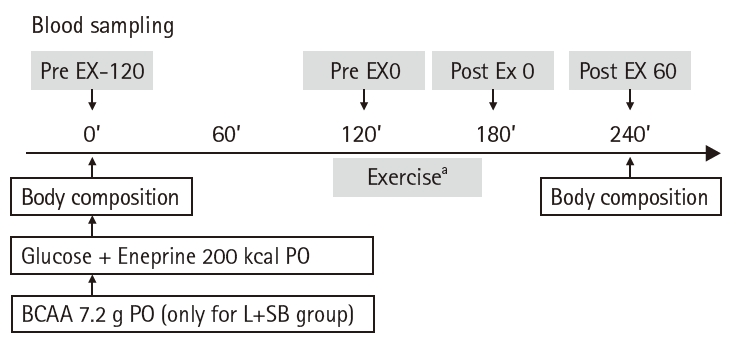
On the day before the clinical trial, the patients fasted overnight with no food or water intake after 9 p.m. The first blood sample (Pre EX-120) was drawn at 8 a.m. on the day of the clinical trial.
To prevent complications caused by hypoglycemia due to insufficient energy substrate supply during and after exercise, one pack of Eneprine (strawberry flavor; Nisshin OilliO Group, Ltd.) energy supplement (110 kcal/pack), was added immediately after the first blood draw, along with a solution of Japanese Pharmacopoeia dextrose in 100 mL of warm water (90 kcal/pack) and a solution of glucose (90 kcal) dissolved in 100 mL of warm water, for a total of 200 kcal for each group of subjects.
The nutrient composition of Eneprine, 40 g/pack contains 110 kcal, 0 g of protein, 9.5 g of fat (including 4.0 g of medium-chain fatty acid oil), 5.4 g of carbohydrate, and 0.6 g of dietary fiber.
Interventions
Clinical trial protocols
The protocol over time on the day of the clinical trial is shown in
Fig. 2. After administration of Eneprine and glucose (for a total of 200 kcal), a second blood sample was drawn 120 minutes after administration (Pre EX 0), when the administered nutrients were considered to have been fully digested and absorbed, followed by 60 minutes of ergometer exercise at 50% intensity of
V˙O
2 max (
Fig. 2). On the day of the study, blood samples were collected 2 hours before the start of exercise (Pre EX-120), 2 hours after the energy administration immediately before the start of exercise (Pre EX 0), and 2 hours after the energy administration immediately after the start of exercise (Pre EX 0), and 60 minutes after the completion of exercise (Post EX 60). Blood biochemical tests included those for levels of serum free-fatty acid (FFA) in μEq/L, blood sugar in mg/dL, various serum amino acids in mmol/mL, and serum insulin levels (INS) in μg/mL.
Exercise load
After 120 minutes of oral intake of 200 kcal of Eneprine and glucose as shown in the clinical study protocol (
Fig. 2),
V˙O
2 max 50% intensity ergometry was conducted. The exercise load lasted for 60 minutes, consisting of 30 minutes on the exercise machine and 30 minutes on the leg press.
Before the day of the clinical trial, maximum oxygen consumption (V˙O2 max, maximum oxygen consumption, in mL/kg·min) was measured on a stationary bicycle (Ergometer Model 828E; Monarch) and the maximum weight on a leg press (Combi) was also measured. A mass spectrometer for biological gas analysis (ARCO-2000; Arco, Inc.) was used for exhaled gas analysis. The ergometer was used to measure heart rate and subjective assessment of exercise intensity was conducted using a subjective exercise intensity scale. The exercise intensity was increased every 4 minutes and ended when the heart rate reached 170 beats per minute or when the subjects reported that the exercise load was unsustainable. The regression line between heart rate and oxygen uptake was drawn and the oxygen uptake at the maximum heart rate, as calculated by “220–age,” was estimated as the VO2 max. In the leg press, the weight was increased step by step starting from a light weight, and the maximum weight that could be lifted (in kg) was set to the weight one step below the weight that could not be lifted at all.
On the day of the clinical study, a total of 60 minutes of exercise was performed, consisting of 30 minutes of exercise at a
V˙O
2 max intensity of 50% as measured by an ergometer and heart rate monitor (Polar) and six sets of 10 repetitions at 70% of one repetition maximum (RM) intensity using a leg press. The degree of exercise load was due to a combination of aerobic exercise using a moderate to high ergometer and resistance exercise using a leg press, and was set at an intensity that could be expected to increase skeletal muscle strength and skeletal muscle mass through repeated exercise [
17]. The subjects were asked to consume water as needed to prevent dehydration before and after the exercise load at their own discretion.
Randomized allocation and propensity score matching were not employed. Instead, group allocation was performed according to baseline clinical characteristics.
Blinding (masking)
No blinding was done.
Outcome variables
Outcome variables were serum FFA and blood glucose levels, serum INS before and after exercise, and amino acid levels before and after exercise.
Data sources/measurement
Data sources were participants’ demographic and clinical findings, and laboratory results. Body composition was measured using the InBody S10 (Inbody Japan Inc.) with a multi-frequency bioimpedance method after the first blood sample (Pre EX-120) on the day of the clinical study. The results are shown in
Table 2. No significant differences in body composition values were evident between the groups.
Since no randomized allocation or propensity matching was done, there may be selection bias and confounding, potentially affecting the comparability between groups.
Study size
Sample size estimation was not done since only available volunteers were included in the study.
Unit of analysis
The unit of analysis was the same as the unit of assignment.
Statistical analysis
All data are presented as the mean±standard error of the mean. An unpaired Student t-test was used for each blood biochemistry value in each group, and a P-value<0.05 was considered indicative of a significant difference. A paired Student t-test was used to compare the values at each blood collection point in the same group, and P<0.05 was considered a significant difference. Statistical significance was tested using Microsoft Excel version 1811.
Results
Background of clinical study subjects
The background of the subjects in each group is shown in
Table 1. There were no significant differences in age, height, weight, body mass index,
V˙O
2 max, and 1 RM between the groups, and no significant differences in subject backgrounds, including exercise habits and exercise capacity, were observed between subjects in each group.
The body composition value for each group on the day of the clinical trial before the oral intake is shown in
Table 2. There was no difference of the body composition value between each group.
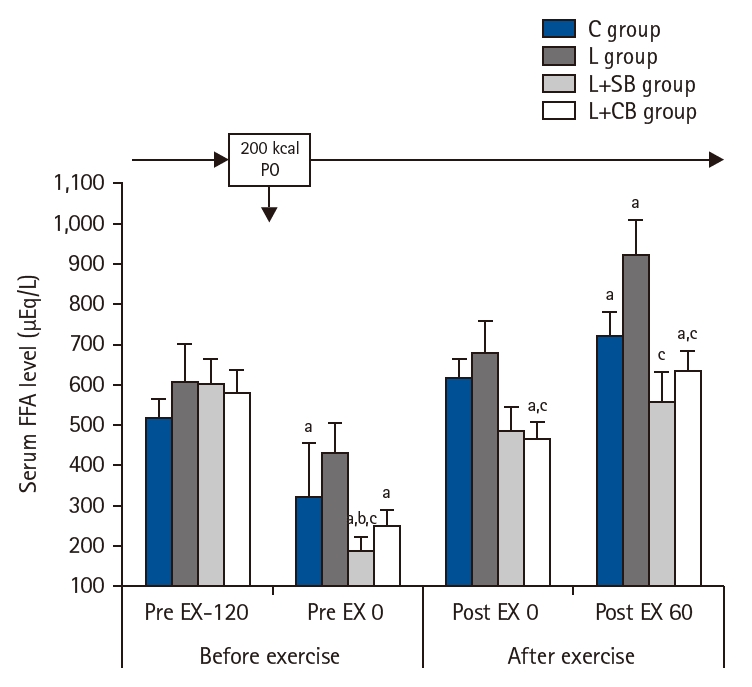
Serum FFA levels at Pre EX-120 in L, L+SB, and L+CB groups were higher than those in C group, but the difference was not statistically significant (
Fig. 3). Serum FFA levels in C, L+SB, and L+CB groups at Pre EX 0 were lower than those at Pre EX-120 (P<0.05). The values in L+SB and L+CB groups were lower than those in C and L groups, and there was a significant difference in L+SB group (P<0.05). While serum FFA levels at Post EX 0 and Post EX 60 in groups C and L were higher than those before the exercise, the differences were not statistically significant. Serum FFA levels in C and L groups at Post EX 0 and Post EX 60 were higher than those before exercise, but the difference was not statistically significant. Serum FFA levels in L+CB group at Post EX 0 were significantly lower than those in L group (P<0.05), and the values in L+SB and L+CB groups at Post EX 60 were significantly lower than those in L group (P<0.05).
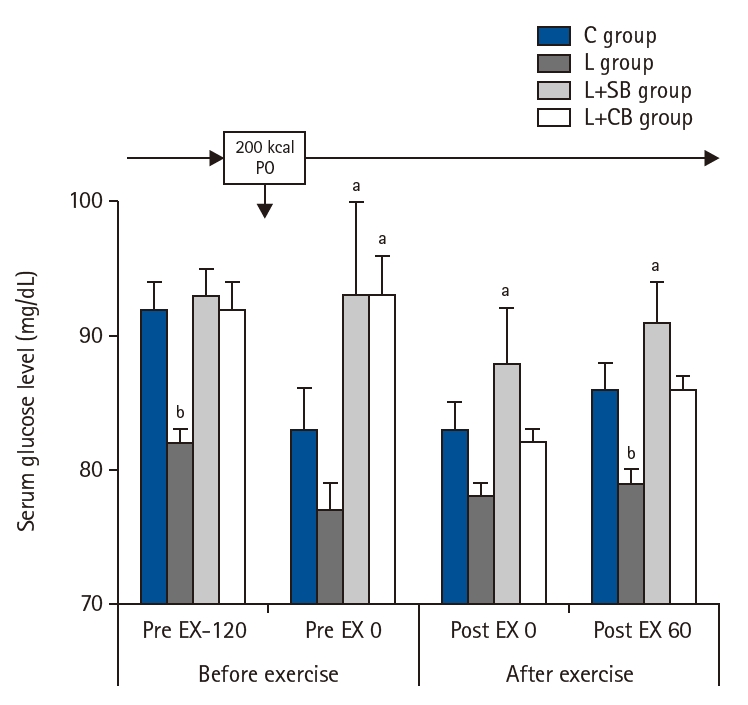
Blood glucose levels of each group were shown in
Fig. 4. Blood glucose levels in L group at Pre EX-120 were significantly lower than those in L+SB and L+CB groups (P<0.05). The values in L+SB and L+CB groups were lower than those in L group at Pre EX 0. At Post EX 0, blood glucose levels in L+SB group were significantly higher than those in L group (P<0.05). At Pot EX 60, the values in L group were significantly lower than those in C, L+SB, and L+CB groups (P<0.05).
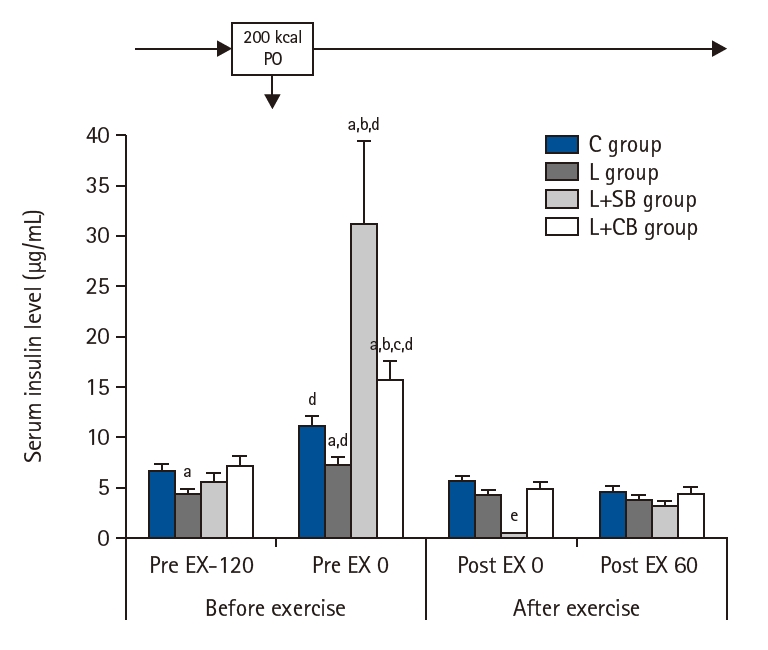
INS before and after exercise in each group are shown in
Fig. 5. The INS of group L was significantly lower than that of group C (P<0.05) before the exercise load and energy administration (Pre EX-120), but the INS of L+SB and L+CB groups were not different from that of C group.
At Pre EX 0, the INS increased significantly in all groups compared with those at Pre EX-120 (P<0.05). The INS in L+SB and L+CB groups were significantly higher than those in L group, and the INS in L+SB groups were significantly higher than those in L+CB group (P<0.05). The INS immediately (Post EX 0) and 60 minutes (Post EX 60) after exercise were significantly lower in all groups, and no difference of the INS between these groups.
Serum amino acid levels before and after exercise in each group
Serum BCAA levels in each group, BCAA/total amino acids, and serum EAA levels are shown in
Table 3. The serum BCAA and EAA levels were significantly lower at Pre EX 0, Post EX 0, and Post EX 60 than those at Pre EX-120 before exercise in all three groups except the L+SB group. Serum BCAA and EAA levels in the L+SB group at Post EX 0 and Post EX 60 were significantly higher at Pre EX 0 and Post EX 0 than at Pre EX-120 before exercise, but there was no difference in values at Post EX 60. Serum BCAA and EAA levels in the L+SB group at Post EX 0 and Post EX 60 were significantly higher than those in the other three groups (P<0.05).
Serum leucine levels in each group are shown in
Table 4. Serum leucine levels for each group were significantly lower at Pre EX 0, Post EX 0, and Post EX 60 than those at Pre EX-120 before exercise in all three groups except the L+SB group (P<0.05). Serum leucine levels in the L+SB group were significantly higher at Pre EX 0, Post EX 0, and Post EX 60 than those at Pre EX-120 before exercise (P<0.05). However, BCAA and EAA values, including those for serum leucine, in the L+CB group at Pre EX 0, Post EX 0, and Post EX 60 were not significantly different from those in the C and L groups.
Discussion
Key results
The blood glucose level and INS in the L+SB and L+CB groups were significantly lower than those in the control group, and the INS in the L+SB group was significantly higher than that in the L+CB group. LCAR alone increased non-oxidative glucose disposal (NOGD), despite a decrease in INS. The decrease in blood glucose despite the decrease in INS suggested that LCAR alone increased NOGD. Furthermore, single and long-term continuous administration of BCAA in addition to LCAR treatment markedly increased INS, suggesting that the ITA of long-term continuous BCAA administration may promote the synthesis of skeletal muscle protein.
Interpretation/comparison with previous researches
It has long been known that a decrease in lean body mass (LBM), such as skeletal muscle mass, correlates closely with prognosis in surgical critical care patients [
18], and recent reports have shown that clinical outcomes are worse in the elderly, particularly when frailty, sarcopenia, and cachexia accompany the loss of biocomponents [
19]. These conditions are known as tissue loss syndrome [
20], but multiple overlaps of these conditions are also common [
19]. All of these conditions are accompanied by a marked decrease in LBM, including skeletal muscle mass, and occur at a high rate in the elderly. Various measures are being used to maintain skeletal muscle mass, including the administration of nutrients and exercise [
15,
17]. Insulin, the most important anabolic hormone that promotes muscle protein synthesis (MPS), reportedly promotes MPS not only under physiological conditions but also under surgical invasive conditions [
21,
22].
LCARs promote fatty acid oxidation by transporting long-chain fatty acids into mitochondria [
23], and the serum FFA values in the LCAR-treated group indicate that the serum FFA values in the LCAR-treated groups were higher than those in the control group, suggesting that LCAR increased lipolysis and fatty acid oxidation.
The results of this study were similar to those of our previous study [
24]. In addition to changes in lipid metabolism, a decrease in blood glucose level and INS was observed after administration of LCAR alone, confirming the effect of LCAR alone on glucose metabolism. Insulin secretion in beta cells, which regulate the oxidation of energy substrates such as glucose and fatty acids, is known to be regulated not only by blood glucose but also by various nutrients such as LCARs and amino acids [
9,
13]. Despite the decrease in INS after administration of LCAR alone compared with to the control group, blood glucose level decreased, suggesting that LCAR alone increased NOGD such as gluconeogenesis and/or glycogen synthesis. Galloway et al. [
13], who performed an oral glucose tolerance test in healthy subjects after long-term continuous administration of LCAR tartrate (3 g/day) for 2 weeks, reported a significant decrease in the area under the concentration-time curve after glucose administration compared with a placebo group. LCAR treatment therefore promotes NOGD, which supports the results of Galloway et al. [
13]. Furthermore, there are many reports suggesting that LCAR administration increases NOGD and increases glycogen storage in skeletal muscle [
25,
26]. The increased glycogen stores in skeletal muscle due to increased NOGD may be advantageous in terms of skeletal muscle energy metabolism and may contribute to improved athletic performance, which may be a benefit of LCAR administration [
27].
In the present study, in addition to LCAR administration, a single dose of BCAA and long-term continuous administration of BCAA were also examined for ITA. The results showed that both the L+SB group and the L+CB group had significantly higher INS compared with the control group, blood glucose level decreased, suggesting that LCAR alone increased NOGD such as gluconeogenesis and/or glycogen synthesis. BCAA administration reportedly promotes insulin secretion and increases MPS, which may be useful in the treatment of type 2 diabetes and sarcopenia [
8]. Among amino acids, leucine, isoleucine, alanine, and arginine are known to promote insulin secretion from beta cells [
9]. Each of these amino acids is believed to exert ITA by different mechanisms [
28]. Leucine, one of the BCAAs, promotes mitochondrial metabolism by activating glutamate dehydrogenase (GDH), which induces insulin secretion from beta cells by activating leucine, and mitochondrial metabolic activity by activating GDH.
Leucine increases ATP/ADP ratio due to an increase in ATP production by the amino group transfer from leucine to α-ketoisocaproic acid, and enters into the TCA cycle via acetyl CoA, resulting in K
ATP channel–dependent depolarization of the beta cell membrane, which has an insulinotropic effect [
29-
31]. Studies using isolated beta cells have also confirmed isoleucine-induced ITA [
32]. However, ITA induced by leucine has been reported to be suppressed in hyperglycemia [
33], indicating that ITA induced by leucine is closely related to glucose metabolism. Because the blood glucose level in the L group was significantly lower than that in the control group due to the increase in NOGD, ITA induced by leucine in combination with BCAA may occur without suppression, resulting in a marked increase in serum INS after energy administration. In the L+SB group, BCAA was administered together with energy administration 2 hours before the exercise load, resulting in a marked increase in serum BCAA levels followed by a marked increase in INS 2 hours later. BCAA administration in the L+CB group was terminated 2 days before the clinical trial, and serum BCAA levels at Pre EX 0 (2 hours after energy administration) were not different from those in the other three groups, including the C group. Nevertheless, INS at 2 hours after energy administration (Pre EX 0) did not differ from that in the L group.
It is noteworthy that the ITA after energy administration in the L+SB group was significantly higher than that in the control group and the L group, although it did not reach the INS in the L+SB group. However, the results of this study showed that prolonged BCAA administration for 14 days up to 2 days before the clinical study had the same potentiating effect on ITA as that of immediate BCAA administration. Even if the serum BCAA level was not high, the ITA-enhancing effect was observed as late as 2 days after the BCAA was administered for a prolonged period.
The mechanism by which ITA is enhanced after energy administration, even in the absence of high serum BCAA levels, is unknown. The BCAAs used in this study are supplements containing EAAs fortified with leucine [
15,
16]. These EAAs are necessary for the synthesis of skeletal muscle protein and are essential substrates for protein anabolism [
34]. It is not clear whether the administration of EAAs such as BCAAs alone promotes skeletal MPS in humans, although there are reports in animal studies [
35]. Protein anabolism is believed to be mediated by ITA rather than by the administration of the amino acids themselves [
36], and ITA is considered an essential response for promoting protein anabolism.
The long-term persistence of high serum BCAA levels after repeated administration may increase the sensitivity of ITA to energy administration, which may be advantageous in condi-tions such as diabetes, in which an increase in insulin secretion is expected. In addition, an increased insulin secretory response to energy administration is advantageous for increased MPS in skeletal muscle. This suggests that ITA is enhanced by long-term continuous BCAA administration and that insulin-induced increases in MPS, and therefore skeletal muscle mass, can be expected. MPS was not measured in this study, and although ITA enhancement was confirmed, the effect on MPS is unknown and should be further investigated in the future.
In general, after exercise at 50% of VO2 max, stress hormones such as serum catecholamine levels increased markedly in response to the marked increase in energy demand due to exercise, suggesting that lipolysis was markedly enhanced and used as an energy substrate. As a result, the serum INS, which is a potent inhibitory hormone of lipolysis, was low in all clinical study groups. There was no difference in INS between the groups when INS in each group was markedly decreased after exercise. The present findings did not show any clear results concerning the effects of LCAR associated with BCAAs as long as the condition after exercise.
Limitations
First, the non-randomized design and lack of propensity score matching may have introduced selection bias and unmeasured confounding, potentially affecting group comparability. Second, the small sample size and inclusion of only healthy young women limit the generalizability to other populations, such as men or older adults. Third, the intervention period was short, and long-term effects of L-carnitine and BCAA were not assessed. Fourth, MPS and physical performance were not directly measured. Finally, self-reported dietary and activity adherence may have introduced variability.
Clinical implications
The findings of this study suggest that both single and prolonged administration of BCAA, when combined with L-carnitine supplementation, significantly potentiate insulin secretion following energy intake in healthy young women. Enhanced ITA, even after discontinuation of BCAA administration, indicates a potential for sustained metabolic benefit. This may have important clinical implications for nutritional strategies aimed at improving glucose regulation and preventing sarcopenia, particularly in populations at risk for muscle loss or impaired insulin sensitivity, such as the elderly or individuals with diabetes.
Conclusion
This study demonstrated that oral administration of L-carnitine, either alone or in combination with BCAA, enhances the insulinotropic response to energy intake in healthy young women. Notably, both single and prolonged BCAA supplementation potentiated insulin secretion, with effects persisting even after cessation of BCAA intake.
Authors’ contribution
Conceptualization: YS, HN, SO. Methodology: YS, HN. Project administration: YS, HN. Writing-original draft: YS. Writing-review & editing: HN, SO. All authors read and approved the final manuscript.
Conflict of interest
Satoshi Odo was employed by Lonza, Tokyo, Japan, which may be perceived as a potential conflict of interest. However, the company had no role in the design, data collection, analysis, or preparation of this manuscript. The other authors of this manuscript have no conflicts of interest to disclose.
Funding
This study was funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Grant-in-Aid for Scientific Research (C), entitled “Research on the combined administration of L-carnitine and branched-chain amino acids for the prevention of sarcopenia in the elderly” (Project #26460917).
Data availability
Contact the corresponding author for research data availability.
Acknowledgments
The authors would like to express their deepest gratitude to Mayumi Nagata, Assistant Professor, Department of Health and Nutrition, Wayo Women’s University, for her invaluable assistance in the preparation and processing of blood samples for the study protocol.
Supplementary materials
None.
Fig. 1.Protocols for each group of clinical trials. LCAR, L-carnitine; BCAA, branched-chain amino acids; C group, control; L group, L-carnitine alone; L+SB group, L-carnitine+single dose of BCAA; L+CB group, L-carnitine+continuous dose of BCAA; exp., experiment.
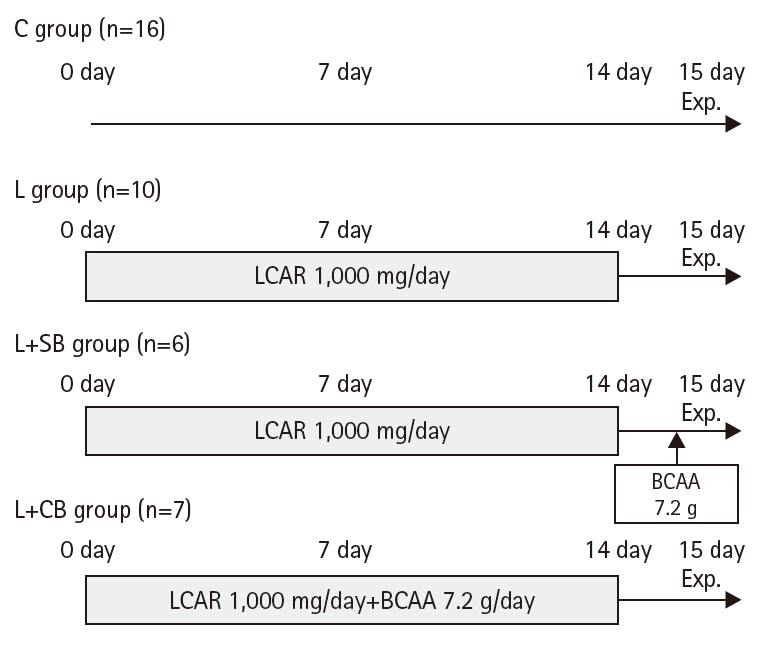
Fig. 2.Protocol on the day of the clinical study. Condition for the clinical study: overnight fasting (no oral intake after 9 PM of 1 day before the clinical study). Pre EX-120, 2 hours before the exercise; Pre EX 0, just before the start of the exercise; Post EX 0, immediately after exercise; Post EX 60, 1 hour after the exercise; PO, per os (by mouth); BCAA, branched-chain amino acids. aExercise: 50% V˙O2 max for 60 minutes.
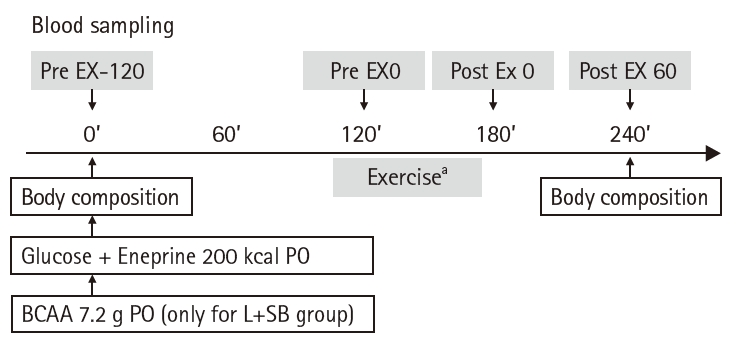
Fig. 3.Serum free-fatty acid (FFA) values before and after exercise in each group. Pre EX-120, 2 hours before the exercise; Pre EX 0, just before the start of the exercise; Post EX 0, immediately after exercise; Post EX 60, 1 hour after the exercise; C group, control; L group, L-carnitine alone; L+SB group, L-carnitine+single dose of BCAA; L+CB group, L-carnitine+continuous dose of BCAA; BCAA, branched-chain amino acids; PO, per os (by mouth); aP<0.05 vs. Pre EX-120; bP<0.05 vs. C group; cP<0.05 vs. L group by paired t-test.
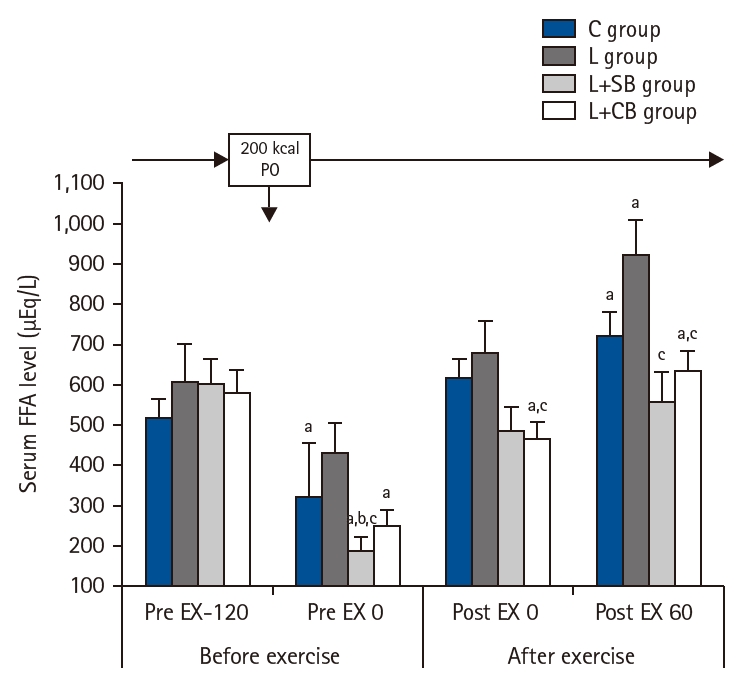
Fig. 4.Blood glucose levels before and after exercise loading in each group. Pre EX-120, 2 hours before the exercise; Pre EX 0, just before the start of the exercise; Post EX 0, immediately after exercise; Post EX 60, 1 hour after the exercise; C group, control; L group, L-carnitine alone; L+SB group, L-carnitine+single dose of BCAA; L+CB group, L-carnitine+continuous dose of BCAA; BCAA, branched-chain amino acids; PO, per os (by mouth); aP<0.01 vs. C, L+SB, L+CB group; bP<0.05 vs. L group by paired t-test.
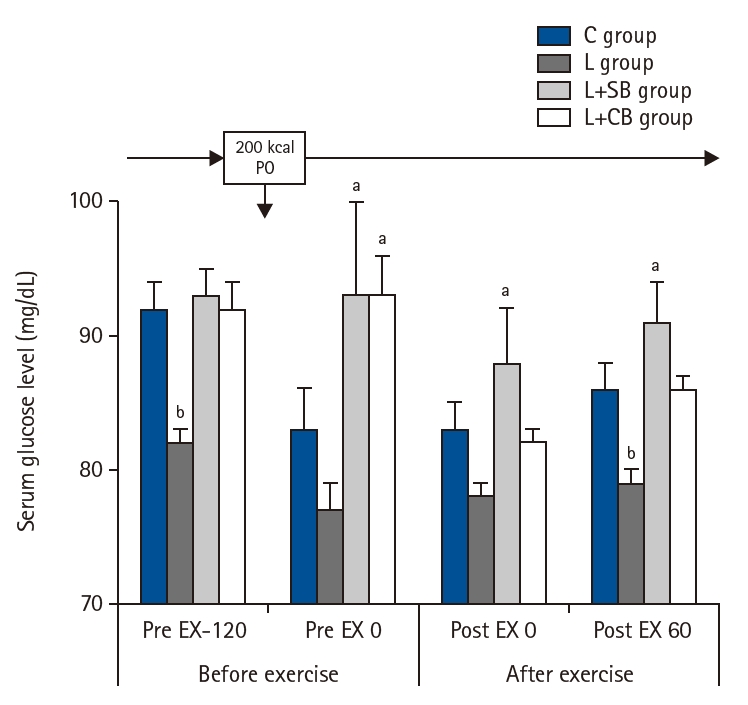
Fig. 5.Serum insulin levels before and after exercise loading in each group. Pre EX-120, 2 hours before the exercise; Pre EX 0, just before the start of the exercise; Post EX 0, immediately after exercise; Post EX 60, 1 hour after the exercise; C group, control; L group, L-carnitine alone; L+SB group, L-carnitine+single dose of BCAA; L+CB group, L-carnitine+continuous dose of BCAA; BCAA, branched-chain amino acids; PO, per os (by mouth). aP<0.05 vs. C group; bP<0.05 vs. L group; cP<0.05 vs. L+SB group; dP<0.05 vs. Pre Ex-120; eP<0.05 vs. L+CB group.
Table 1.Background factors for each group
|
C group (n=16) |
L group (n=10) |
L+SB group (n=6) |
L+CB group (n=7) |
|
Age (yr) |
20.8±0.2 |
20.9±0.1 |
20.7±0.2 |
20.4±0.2 |
|
Height (cm) |
160.5±1.5 |
159.5±1.8 |
157.2±1.6 |
161.9±1.3 |
|
Body weight (kg) |
57.0±1.7 |
55.8±1.7 |
53.8±4.4 |
54.7±2.7 |
|
Body mass index (kg/m²) |
21.8±0.5 |
22.2±0.7 |
21.6±1.4 |
20.8±0.8 |
|
V˙O max (mL/min) |
35.3±2.5 |
31.1±2.3 |
28.7±2.7 |
35.3±3.2 |
|
1 RM (kg) |
117.2±9.4 |
121.7±9.8 |
107.6±8.5 |
112.6±12.3 |
Table 2.Body composition of each group
|
C group (n=16) |
L group (n=10) |
L+SB group (n=6) |
L+CB group (n=7) |
|
Body mass index (kg/m²) |
21.8±0.5 |
22.2±0.7 |
21.6±1.3 |
20.8±0.8 |
|
ICW (L) |
18.7±0.6 |
17.6±0.5 |
16.5±0.8 |
19.3±0.9 |
|
ECW (L) |
11.1±0.4 |
10.8±0.3 |
9.8±0.5 |
11.6±0.4 |
|
TBW (L) |
29.8±1.0 |
28.5±0.8 |
26.2±1.3 |
30.9±1.4 |
|
ECW/TBW |
0.4±0.0 |
0.4±0.0 |
0.4±0.0 |
0.4±0.0 |
|
Muscle weight (kg) |
38.3±1.2 |
36.5±1.0 |
33.8±1.7 |
39.8±1.8 |
|
%Fat (%) |
27.9±1.1 |
30.9±1.5 |
32.4±2.1 |
22.1±1.8 |
|
LBM (kg) |
40.8±1.3 |
38.9±1.1 |
35.9±2.1 |
42.5±1.9 |
Table 3.Serum BCAA levels, BCAA/total AA, and serum EAA levels for each group
|
BCAA |
BCAA/total AA |
EAA |
|
Pre EX-120 |
Pre EX 0 |
Post EX 0 |
Post EX 60 |
Pre EX-120 |
Pre EX 0 |
Post EX 0 |
Post EX 60 |
Pre EX-120 |
Pre EX 0 |
Post EX 0 |
Post EX 60 |
|
C group (n=16) |
367±9 |
263±9a
|
296±10 |
294±16 |
0.15±0.01 |
0.12±0.01a
|
0.12±0.01 |
0.12±0.00 |
869±22 |
665±20a
|
725±19 |
701±18 |
|
L group (n=10) |
378±18 |
274±17a
|
295±13 |
294±16 |
0.15±0.01 |
0.13±0.01a
|
0.13±0.01 |
0.13±0.01 |
858±23 |
662±24a
|
696±20 |
717±20 |
|
L+SB group (n=6) |
364±15 |
862±51a,b
|
520±37b
|
411±42b
|
0.15±0.01 |
0.29±0.01a,b
|
0.20±0.01a,b
|
0.17±0.01 |
848±33 |
1,539±72a,b
|
1,066±45b
|
893±62 |
|
L+CB group (n=7) |
361±21 |
249±17a |
289±16 |
287±18 |
0.14±0.01 |
0.12±0.01a
|
0.12±0.01 |
0.12±0.01 |
854±33 |
642±27a
|
717±30 |
719±34 |
Table 4.Serum leucine levels in each group
|
Group |
Pre EX-120 |
Pre EX 0 |
Post EX 0 |
Post EX 60 |
|
C group (n=16) |
111±3 |
72±3a
|
85±3 |
86±3 |
|
L group (n=10) |
112±7 |
75±5a
|
88±4 |
91±3 |
|
L+SB group (n=6) |
107±3 |
421±28a,b
|
246±22b
|
198±20b
|
|
L+CB group (n=7) |
106±5 |
68±4a
|
85±4 |
86±5 |
References
- 1. Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in communitydwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16-23. ArticlePubMed
- 2. Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 2010;11:391-6. ArticlePubMedPMC
- 3. Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984;7:465-70. ArticlePubMed
- 4. Gannon MC, Nuttall FQ, Lane JT, Burmeister LA. Metabolic response to cottage cheese or egg white protein, with or without glucose, in type II diabetic subjects. Metabolism 1992;41:1137-45. ArticlePubMed
- 5. Gannon MC, Nuttall FQ, Grant CT, Ercan-Fang S, Ercan-Fang N. Stimulation of insulin secretion by fructose ingested with protein in people with untreated type 2 diabetes. Diabetes Care 1998;21:16-22. ArticlePubMedPDF
- 6. Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:157-62. ArticlePubMedPMCPDF
- 7. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 2011;8:68.Article
- 8. Manders RJ, Little JP, Forbes SC, Candow DG. Insulinotropic and muscle protein synthetic effects of branched-chain amino acids: potential therapy for type 2 diabetes and sarcopenia. Nutrients 2012;4:1664-78. ArticlePubMedPMC
- 9. Newsholme P, Brennan L, Rubi B, Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin Sci (Lond) 2005;108:185-94. ArticlePubMed
- 10. Hoppel CL, Genuth SM. Carnitine metabolism in normalweight and obese human subjects during fasting. Am J Physiol 1980;238:E409-15. ArticlePubMed
- 11. Soeters MR, Sauerwein HP, Duran M, Wanders RJ, Ackermans MT, Fliers E, et al. Muscle acylcarnitines during short-term fasting in lean healthy men. Clin Sci (Lond) 2009;116:585-92. ArticlePubMedPDF
- 12. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1-8. ArticlePubMedPMC
- 13. Galloway SD, Craig TP, Cleland SJ. Effects of oral Lcarnitine supplementation on insulin sensitivity indices in response to glucose feeding in lean and overweight/obese males. Amino Acids 2011;41:507-15. ArticlePubMedPDF
- 14. Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695-700. ArticlePubMedPMCPDF
- 15. Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab 2015;308:E1056-65. ArticlePubMed
- 16. Kato H, Suzuki H, Mimura M, Inoue Y, Sugita M, Suzuki K, et al. Leucine-enriched essential amino acids attenuate muscle soreness and improve muscle protein synthesis after eccentric contractions in rats. Amino Acids 2015;47:1193-201. ArticlePubMedPMCPDF
- 17. Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007;116:572-84. ArticlePubMed
- 18. Kinney JM. Metabolic response to injuries. In: Winters RW, Greene HL, editors. Nutritional support of the seriously ill patient. Academic Press; 1983. p. 5-12.
- 19. Sakurai Y. Tissue loss syndrome as an important predictor of survival and clinical outcome in surgical patients: the importance of frail assessment and frail index. Japanese J Surg Metab Nutr 2020;54:109-18. ArticlePubMed
- 20. Gingrich A, Volkert D, Kiesswetter E, Thomanek M, Bach S, Sieber CC, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr 2019;19:120.ArticlePubMedPMCPDF
- 21. Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811-9. ArticlePubMedPMC
- 22. Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg 1995;222:283-97. ArticlePubMedPMC
- 23. Pekala J, Patkowska-Sokoła B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S, et al. L-carnitine: metabolic functions and meaning in humans life. Curr Drug Metab 2011;12:667-78. ArticlePubMed
- 24. Sakurai Y, Hasegawa Y, Kurosaka Y, Nanba H, Odo S. Effects of L-carnitine and branched-chain amino acids on energy metabolism, body composition, and delayed-onset muscle soreness after exercise in healthy subjects. J Nutr Oncol 2018;3:25-33.
- 25. Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab 2006;91:5013-8. ArticlePubMed
- 26. Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 2007;581(Pt 2):431-44. ArticlePubMedPMC
- 27. Odo S. Effect of L-carnitine intake on athletic performance. Jpn J Surg Metab Nutr 2020;54:81-4.
- 28. Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes 2006;55(Suppl 2):S39-47. ArticlePDF
- 29. Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 1980;288:187-9. ArticlePubMedPDF
- 30. Gylfe E. Comparison of the effects of leucines, non-metabolizable leucine analogues and other insulin secretagogues on the activity of glutamate dehydrogenase. Acta Diabetol Lat 1976;13:20-4. ArticlePubMedPDF
- 31. Panten U, Kriegstein E, Poser W, Schonborn J, Hasselblatt A. Effects of L-leucine and alpha-ketoisocaproic acid upon insulin secretion and metabolism of isolated pancreatic islets. FEBS Lett 1972;20:225-8. ArticlePubMed
- 32. Sener A, Somers G, Devis G, Malaisse WJ. The stimulussecretion coupling of amino acid-induced insulin release. Biosynthetic and secretory responses of rat pancreatic islet to L-leucine and L-glutamine. Diabetologia 1981;21:135-42. ArticlePubMed
- 33. MacDonald MJ, McKenzie DI, Kaysen JH, Walker TM, Moran SM, Fahien LA, et al. Glucose regulates leucineinduced insulin release and the expression of the branched chain ketoacid dehydrogenase E1 alpha subunit gene in pancreatic islets. J Biol Chem 1991;266:1335-40. ArticlePubMed
- 34. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250-8. ArticlePubMedPMC
- 35. Wolfe RR. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J Int Soc Sports Nutr 2017;14:30.ArticlePubMedPMCPDF
- 36. Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J 1988;254:579-84. ArticlePubMedPMCPDF

 , Hideyuki Namba2
, Hideyuki Namba2 , Satoshi Odo3
, Satoshi Odo3







 E-submission
E-submission KSPEN
KSPEN KSSMN
KSSMN ASSMN
ASSMN JSSMN
JSSMN Cite
Cite